
Concept explainers
(a)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
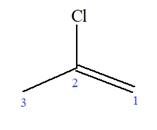
Explanation of Solution
The given molecule is
In this molecule, the root is propene. Thus, the longest carbon chain must have three carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C1 and C2.
The root can be shown as:
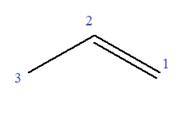
At C2 carbon atom of the root, one chlorine is attached. Thus, the structure of
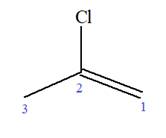
The structure of
(b)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
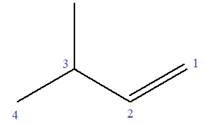
Explanation of Solution
The given molecule is
In this molecule, the root is butene. Thus, the longest carbon chain must have four carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C1 and C2.
The root can be shown as:
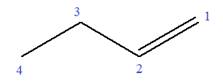
At C3 carbon atom of the root, a methyl substituent is attached.
Thus, the structure of

The structure of
(c)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
Explanation of Solution
The given molecule is
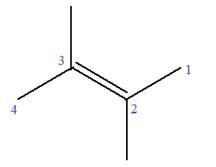
In this molecule, the root is butene. Thus, the longest carbon chain must have four carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C2 and C3.
The root can be shown as:
At C2 and C3 carbon atoms of the root, two methyl substituents are attached.
Thus, the structure of
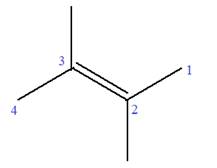
The structure of
(d)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents, are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
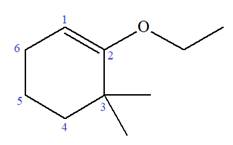
Explanation of Solution
The given molecule is
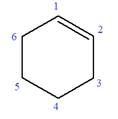
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have six carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2 and C3 carbon atoms of the ring, one ethoxy and two methyl substituents are attached respectively. Thus, the structure of

The structure of
(e)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
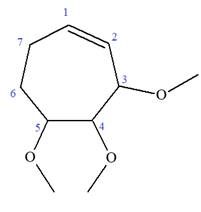
Explanation of Solution
The given molecule is
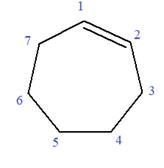
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have seven carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2, C3, and C4 carbon atoms of this ring, three methoxy substituents are attached.
Thus, the structure of
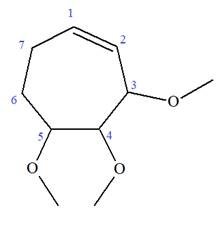
The structure of
(f)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
Explanation of Solution
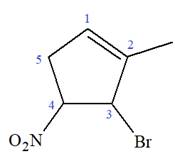
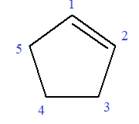
The given molecule is
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have six carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2, C3, and C4 carbon atoms, bromine, methyl, and nitro group are attached.
Thus, the structure of
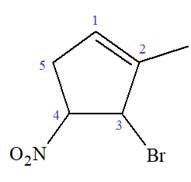
The structure of
(g)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
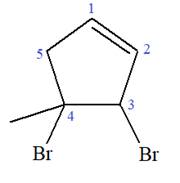
Explanation of Solution
The given molecule is
In this molecule, the root is cyclopentene. Thus, the largest carbon ring must have five carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
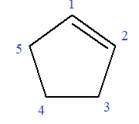
At C3 and C4 carbon atoms of the ring, two bromine atoms and one methyl group are attached.
Thus, the structure of
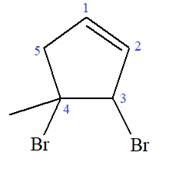
The structure of
(h)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
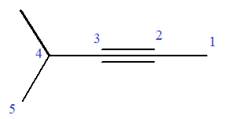
Explanation of Solution
The given molecule is
In this molecule, the root is pentyne. Thus, the longest carbon chain must have five carbon atoms. The suffix ‘yne’ indicates that there is a triple bond in the chain. The position of the triple bond in the chain is always between carbon atoms C2 and C3.
The root can be shown as:
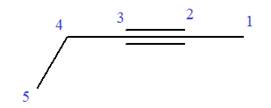
At C4 carbon atom of the root, a methyl group is attached.
Thus, the structure of
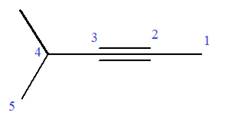
The structure of
Want to see more full solutions like this?
Chapter B Solutions
ORGANIC CHEMISTRY PRINCIPLES & MECHANISM
- How does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forwardBenzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forward
- Draw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forward
- pls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





