
Concept explainers
(a)
Interpretation:
The description of the molecule shown, having G as a generic substituent, is to be written.
Concept introduction:
An
Answer to Problem A.1P
The given molecule is a substituted ethane.
Explanation of Solution
The given molecule is:
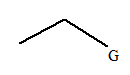
In this molecule, there are two carbon atoms in the straight chain. Hence, the alkane must be ethane
The given molecule is a substituted ethane.
(b)
Interpretation:
The description of the molecule shown, having G as a generic substituent, is to be written.
Concept introduction:
An alkane is said to be substituted if a hydrogen atom of the alkane is replaced by another atom or group of atoms. The atom or group of atoms which replaces the hydrogen atom is called a substituent. It is shown by G, which means a generic substituent.
Answer to Problem A.1P
The given molecule is a substituted pentane.
Explanation of Solution
The given molecule is:
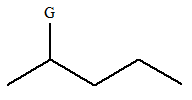
In this molecule, there are five carbon atoms in the straight chain. Hence, the alkane must be pentane
The given molecule is a substituted pentane.
Want to see more full solutions like this?
Chapter A Solutions
Get Ready for Organic Chemistry
- Draw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forwardDraw the monomers required to synthesize this condensation polymer please.arrow_forward
- Provide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forwardIdentify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forward
- H- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform has absorption bands at: 350 nm (absorbance A = 2.34); 514 nm(absorbance A = 0.0532); Calculate the molar absorptivity values for these bands. Comment on their possible nature (charge transfer transitions or d-d S N- transitions?). (4 points)arrow_forward
- What is the mechanism for this?arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward
