
Concept explainers
Interpretation:
The structure of the compound with molecular formula
Concept introduction:
Mass spectrometry is a technique used for measuring the masses of atoms and molecules with great accuracy.
In a mass spectrometer, the vapor of organic compound is bombarded with a beam of high energy electrons that makes the neutral molecule lose an electron and converts it to a radical cation known as a molecular ion.
In
In
Nuclear Magnetic Resonance (NMR) is one of the most capable analytical techniques used for determining the
Few elements, such as
In
Induced magnetic field consists of electricity generated from movement in a magnetic field.
The position of a signal on x-axis in the
The number of signals in
The area covered by the signal is proportional to the number of equivalent protons causing the signal.
The hydrogen atoms on adjacent carbon atoms split the signal into two or more peaks. One, two or three hydrogen atoms split the signal into two, three or four peaks described as doublet, triplet or quartet respectively.
A decrease in the electron density around a proton deshields the signal downfield at a larger value of chemical shift.
An increase in electron density shields the signal upfield at a lower value of chemical shift.
The peak at
The peak at
The peak at
Answer to Problem 1PP
Solution: The structure of the compound with molecular formula
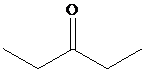
Explanation of Solution
The figure given below represents the
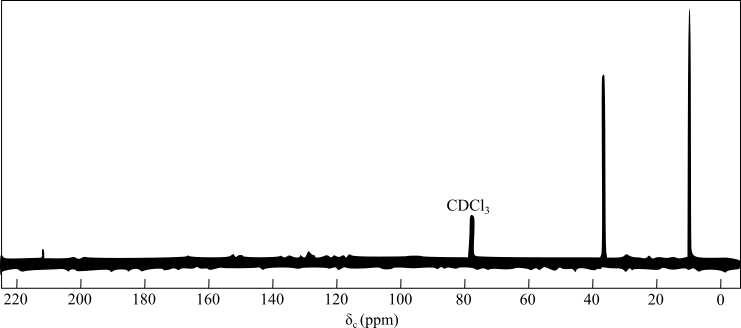
In DEPT
The peak at
The peak at
The peak at
The presence of two signals in the alkyl group region indicates the presence of symmetry in the compound and the two unique carbon atoms.
Therefore, the structure of the compound is as:

The structure of the compound with molecular formula

Want to see more full solutions like this?
Chapter A Solutions
ORGANIC CHEMISTRY-WILEYPLUS ACCESS PKG.
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
