
(a)
Interpretation:
The probability of the presence of an electron at nucleus A in the bonding and also in the antibonding orbitals is to be calculated.
Concept introduction:
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbital of an atom. The distribution of the electrons in the molecules is described by the molecular orbital theory. The unoccupied molecular orbital having lowest energy is known as the LUMO and the occupied molecular orbital having highest energy is known as the HOMO.
(a)
Answer to Problem 9B.2P
The probability of the presence of an electron at nucleus A in the bonding orbital is
Explanation of Solution
It is given that a small-electron probe having volume
The value of R is
The value of
The value of
The expression that is used represent probability of finding an electron is given below.
Where,
The distance between A and B is
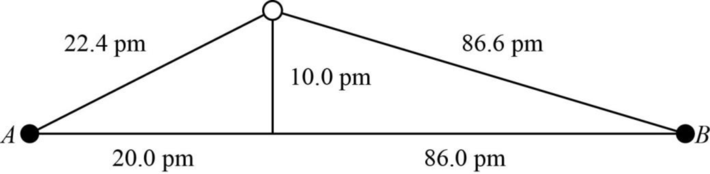
Figure 1
The value of
Substitute the values of
Substitute the values of
Therefore, the probability of the presence of an electron at nucleus A in the bonding orbital is
(b)
Interpretation:
The probability of the presence of an electron at nucleus B in the bonding and also in the antibonding orbitals is to be calculated.
Concept introduction:
Refer to part (a).
(b)
Answer to Problem 9B.2P
The probability of the presence of an electron at nucleus B in the bonding orbital is
Explanation of Solution
It is given that a small-electron probe having volume
The value of R is
The value of
The value of
The expression that is used represent probability of finding an electron is given below.
Where,
The distance between A and B is
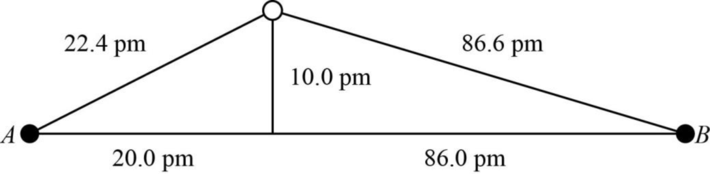
Figure 1
The value of
Substitute the values of
Substitute the values of
Therefore, the probability of the presence of an electron at nucleus B in the bonding orbital is
(c)
Interpretation:
The probability of the presence of an electron at half way between A and B in the bonding and also in the antibonding orbitals is to be calculated.
Concept introduction:
Refer to part (a).
(c)
Answer to Problem 9B.2P
The probability of the presence of an electron at half way between A and B in the bonding orbital is
Explanation of Solution
It is given that a small-electron probe having volume
The value of R is
The value of
The value of
The expression that is used represent probability of finding an electron is given below.
Where,
The distance between A and B is
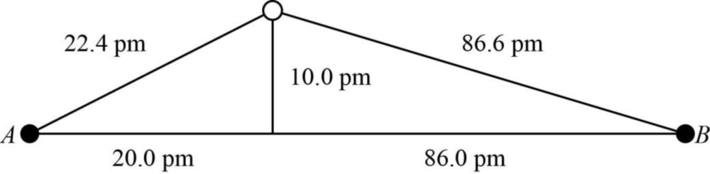
Figure 1
The value of
Substitute the values of
Substitute the values of
Therefore, the probability of the presence of an electron at half way between A and B in the bonding orbital is
(d)
Interpretation:
The probability of the presence of an electron at a point
Concept introduction:
Refer to part (a).
(d)
Answer to Problem 9B.2P
The probability of the presence of an electron at a point
Explanation of Solution
It is given that a small-electron probe having volume
The value of R is
The value of
The value of
The expression that is used represent probability of finding an electron is given below.
Where,
The distance between A and B is
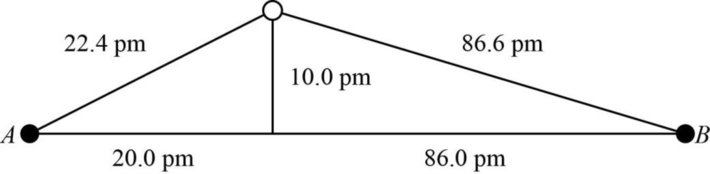
Figure 1
The value of
Substitute the values of
Substitute the values of
Therefore, the probability of the presence of an electron at a point
Want to see more full solutions like this?
Chapter 9 Solutions
ATKINS' PHYSICAL CHEMISTRY
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





