
Concept explainers
(a)
Interpretation:
The stronger of the given pair of nucleophiles in ethanol is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. Generally a negatively charged species is a stronger nucleophile than an uncharged species. In a charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is less stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. If the atoms carrying the charge are from the same group, the order of strength is reversed in a protic solvent because the solvation is greater for smaller atoms.
Answer to Problem 9.57P
The stronger nucleophile of the two is
Explanation of Solution
The given pair of nucleophiles is
Negatively charged nucleophiles are stronger than corresponding uncharged nucleophiles. Of the two nucleophiles, the second
Therefore,
Negatively charged species are stronger nucleophiles than uncharged ones.
(b)
Interpretation:
The stronger of the given pair of nucleophiles, in ethanol, is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. Generally a negatively charged species is a stronger nucleophile than an uncharged species. In the charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is less stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. If the atoms carrying the charge are from the same group, the order of strength is reversed in a protic solvent because the solvation is greater for smaller atoms.
Answer to Problem 9.57P
The stronger nucleophile of the two is
Explanation of Solution
The given pair of nucleophiles is
Both are uncharged species, and the donor atoms in the two are from the same period. In this case, the strength of the nucleophile will depend on the electronegativity of the donor atom. The less electronegative nitrogen atom is better able to donate its lone pair than the more electronegative oxygen atom.
Therefore, the stronger electrophile is
The strength of a nucleophile increases with decreasing electronegativity of the donor atom.
(c)
Interpretation:
The stronger of the given pair of nucleophiles in ethanol is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. Generally a negatively charged species is a stronger nucleophile than an uncharged species. In the charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is less stable on a relatively less electronegative atom Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. If the atoms carrying the charge are from the same group, the order of strength is reversed in a protic solvent because the solvation is greater for smaller atoms.
Answer to Problem 9.57P
The stronger nucleophile of the two is
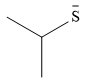
Explanation of Solution
The given pair of nucleophiles is
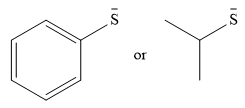
Both species are negatively charged, so the solvation effect of the protic solvent ethanol is expected to be similar.
The donor atom is also the same in both species. The strength as a nucleophile will then depend on the stability of the charge.
In the first species, the charge is resonance stabilized over a total of four atoms.
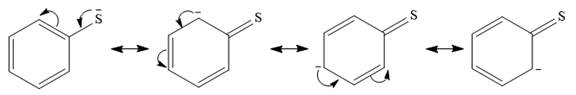
There is no such stabilization in the second species.
Therefore, the stronger nucleophile is the second species.

Resonance stabilization of the charge reduces the strength of a negatively charged nucleophile.
(d)
Interpretation:
The stronger of the given pair of nucleophiles in ethanol is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. Generally a negatively charged species is a stronger nucleophile than an uncharged species. In the charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is less stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. If the atoms carrying the charge are from the same group, the order of strength is reversed in a protic solvent because the solvation is greater for smaller atoms.
Answer to Problem 9.57P
The stronger nucleophile of the two is
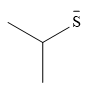
Explanation of Solution
The given pair of nucleophiles is
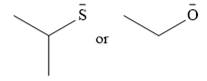
Both are negatively charged nucleophiles with the charge on atoms, S and O, that are from the same group. The sulfur atom is considerably larger than the oxygen atom, and normally would be expected to stabilize the charge better. This would mean that the second nucleophile, with less charge stabilization would be the stronger nucleophile.
However, the solvent in this case, ethanol is protic. It will solvate the smaller oxygen atom much better than the sulfur atom. This solvation will stabilize the second species more than the first one.
Therefore, the stronger nucleophile of the two in ethanol would be

Solvation of negatively charged species by protic solvents can reverse the usual order for nucleophiles containing donor atoms from the same group of the periodic table.
(e)
Interpretation:
The stronger of the given pair of nucleophiles in ethanol is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. Generally a negatively charged species is a stronger nucleophile than an uncharged species. In the charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is less stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. If the atoms carrying the charge are from the same group, the order of strength is reversed in a protic solvent because the solvation is greater for smaller atoms.
Answer to Problem 9.57P
The stronger nucleophile of the two is
Explanation of Solution
The given pair of nucleophiles is
Both are negatively charged species, with donor atoms that carry the charge from the same group. Usually this would mean the species with the smaller donor atom would be the stronger nucleophile. However, the solvent in this case is a protic solvent, ethanol. The smaller sulfur atom will be more strongly solvated than the larger selenium atom. This will reduce the strength of the sulfur containing nucleophile much more than that of the selenium containing nucleophile, thus reversing the usual order.
Therefore, the stronger nucleophile in ethanol is
Solvation of negatively charged species by protic solvents can reverse the usual order for nucleophiles containing donor atoms from the same group of the periodic table.
(f)
Interpretation:
The stronger of the given pair of nucleophiles in ethanol is to be determined.
Concept introduction:
The strength of a nucleophile depends on several factors. Generally, a negatively charged species is a stronger nucleophile than an uncharged species. In the charged nucleophile, the less stable the charge, the higher the strength as a nucleophile. A negative charge is less stable on a relatively less electronegative atom. Therefore, when comparing atoms from the same period, the less electronegative atom (with a negative charge) is a stronger nucleophile. For atoms from the same group, stability of the charge depends on the size of the atom. If the atom bearing the charge is small, the stability is less and strength as a nucleophile is higher. Resonance can stabilize a charge through delocalization over more than one atom, so resonance stabilization of the charge reduces the strength of a nucleophile. The strength also depends, to some extent, on the inductive effect of the groups attached to the carbon that is bonded to the nucleophilic atom. Inductively electron donating groups destabilize the charge and increase the strength of the nucleophile. On the other hand, inductively electron withdrawing groups decrease the strength of a nucleophile.
Protic solvents strongly solvate and stabilize negatively charged species, and therefore, reduce the strength of negatively charged nucleophiles. If the atoms carrying the charge are from the same group, the order of strength is reversed in a protic solvent because the solvation is greater for smaller atoms.
Answer to Problem 9.57P
The stronger nucleophile of the two is
Explanation of Solution
The given pair of nucleophiles is
Both are negatively charged species, with the charge on atoms that are from the same period. The sizes of the two atoms are nearly the same, so solvation by the protic solvent is not expected to differ significantly. The strength will depend mostly on the electronegativity of the atom. The less electronegative Se will not stabilize the charge as strongly as the more electronegative Br.
Therefore, the stronger nucleophile of the two is
For nucleophiles containing donor atoms from the same period, lower the electronegativity of the donor atom, stronger is the nucleophile.
Want to see more full solutions like this?
Chapter 9 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- 20.19 Predict the structure of the major 1,2-addition product formed by reaction of one mole of Cl₂ with 3-methylenecyclohexene. Also predict the structure of the 1,4-addition product formed under these conditions. 20.20 Which of the two molecules shown do you expect to be the major product formed by 1,2-addition of HCI to cyclopentadiene? Explain. Cyclopentadiene + HC 3-Chlorocyclopentene (racemic) or 4-Chlorocyclopentene (racemic)arrow_forward20.35 Propose structural formulas for compounds A and B and specify the configuration of compound B. EtO₂C 250°C C14H2004 CO₂Et 1. Oso, then NaHSO3 2. HIO4 C14H2006 A Barrow_forward20.21 Predict the major product formed by 1,4-addition of HCI to cyclopentadiene. 20.22 Draw structural formulas for the two constitutional isomers with the molecular for- mula C₂H,Br, formed by adding one mole of Br, to cyclopentadiene.arrow_forward
- Add substituents to draw the conformer below (sighting down the indicated bond), then rotate the back carbon to provide the conformation that will be capable of an E2 elimination. R/S stereochemistry is graded. + I I H CH3 Ph Досн Br OCH 3 Drawing Q H Atoms, Bonds and Rings Charges Tap a node to see suggestions. H H H H H Undo Reset Remove Done Rotatearrow_forward20.17 Predict the structure of the major product formed by 1,2-addition of HBr to 3-methylenecyclohexene. 3-Methylenecyclohexene 20.18 Predict the major product formed by 1,4-addition of HBr to 3-methylenecyclohexene.arrow_forward+ Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. Br Drawing Strong Base H Q Atoms, Bonds Charges and Rings Draw or tap a new bond to see suggestions. Remove Done 語 Reset Undo + Drag To Panarrow_forward
- Draw a vicinal alkyl bromide that would produce the following alkene in an E2 elimination. Use a dash or wedge bond to indicate stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. + Drawing Į Strong Base H Br Q Atoms, Bonds and Rings Charges Draw or tap a new bond to see suggestions. Undo Reset 謂 Remove Done Drag To Pan +arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. + Br CH3 Q Strong Base Drawing Atoms, Bonds and Rings Charges Undo Reset H "Br H N Br. Remove Done .N. Drag To Panarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the product of this elementary step in an elimination mechanism. Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore byproducts. + Br: .. 8 0.01 M NaOH heat Drawing Q Atoms, Bonds and Rings Charges and Lone Pairs Draw or tap a new bond to see suggestions. Undo Reset Remove Done + Drag To Panarrow_forward
- + Draw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. Ph CH2CH3 H H3C H Br DBN [૪] Drawing Atoms, Bonds and Rings H | OH Charges ―00 H. C | Undo Reset Br I Remove Done Drag To Pan +arrow_forwardReaction A Now the production A Œ In the product of reaction i 12 Dear the product of actionarrow_forwardMacmillan Learnin When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M* = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm-1. The 13C NMR and DEPT spectra are provided. Draw the structure of the product as the resonance contributor lacking any formal charges. 13C NMR DEPT 90 200 160 120 80 40 0 200 160 120 80 DEPT 135 200 160 120 80 40 0 Draw the unknown amide. 40 40 0arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

