
Concept explainers
(a)
Interpretation:
The products expected when
Concept introduction:
An
All these reactions take place in the presence of basic compounds but in case of
Answer to Problem 9.46AP
The products formed when
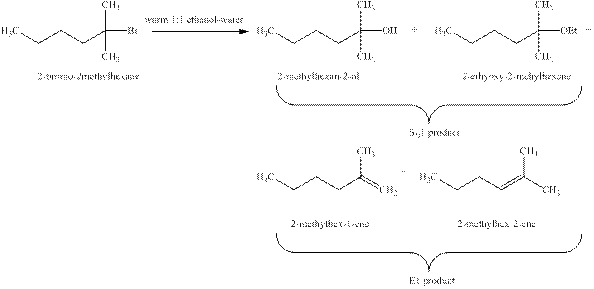
Explanation of Solution
The types of reactions which
The products that are obtained via

Figure 1
The
An
The products formed when
(b)
Interpretation:
The products expected when
Concept introduction:
An alkyl halide in the presence of basic compounds undergoes multiple kinds of reaction, for example,
All these reactions take place in the presence of basic compounds but in case of
Answer to Problem 9.46AP
The products expected when

Explanation of Solution
An
The products formed for this reaction are shown below.

Figure 2
An
The products expected when
(c)
Interpretation:
The products expected when
Concept introduction:
An alkyl halide in the presence of basic compounds undergoes multiple kinds of reaction, for example,
All these reactions take place in the presence of basic compounds but in case of
Answer to Problem 9.46AP
The products expected when
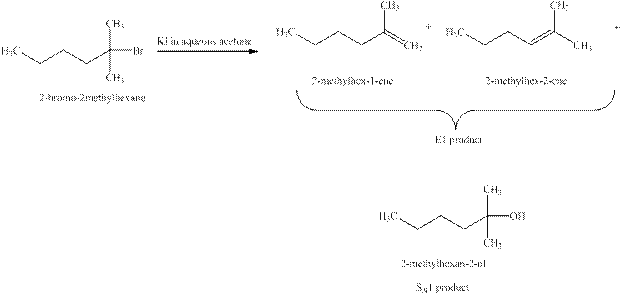
Explanation of Solution
The type of reactions which
The products that are obtained via

Figure 3
The
An
The products expected when
(d)
Interpretation:
The products expected from the reaction of the products of part (b) and
Concept introduction:
An
Answer to Problem 9.46AP
The products expected from the reaction of the products of part (b) and
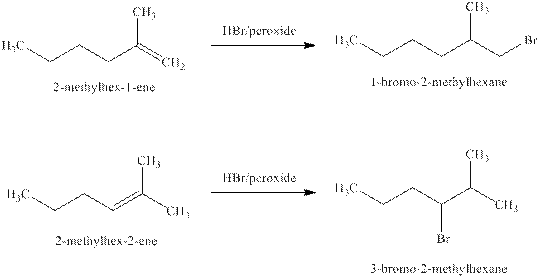
Explanation of Solution
Some reactions follow the rule of Markonikov’s addition but some do not. Markonikov’s gave the rule purely on the basis of the observation of products received by him when performed the addition reaction on alkene.
Some reactions do not follow the rule because they follow the stability of the intermediate formed in the mechanism of that particular reaction.
The reaction of alkenes (
The products expected from the reaction of the products of part (b) and

Figure 4
The products expected from the reaction of the products of part (b) and
(e)
Interpretation:
The products expected from the reaction of the products of part (b) and
Concept introduction:
An alkene undergoes addition reactions due to the presence of unsaturated bond in it. Markonikov’s gave a rule for the addition of protic acids or protic compounds takes place in such a way that proton goes to carbon having the highest number of hydrogen and anion goes to other carbon.
Answer to Problem 9.46AP
The products expected from the reaction of the products of part (b) and
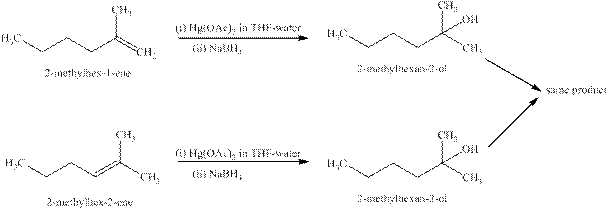
Explanation of Solution
Some reactions follow the rule of Markonikov’s addition but some do not. Markonikov’s gave the rule purely on the basis of the observation of products received by him when performed the addition reaction on alkene.
Some reactions do not follow the rule because they follow the stability of the intermediate formed in the mechanism of that particular reaction.
The reaction of alkenes (
The products expected from the reaction of the products of part (b) and

Figure 5
The products expected from the reaction of the products of part (b) and
(f)
Interpretation:
The products expected from the reaction of the products of part (b) and
Concept introduction:
An alkene undergoes addition reactions due to the presence of unsaturated bond in it. Markonikov’s gave a rule for the addition of protic acids or protic compounds takes place in such a way that proton goes to carbon having the highest number of hydrogen and anion goes to other carbon.
Answer to Problem 9.46AP
The products expected from the reaction of the products of part (b) and
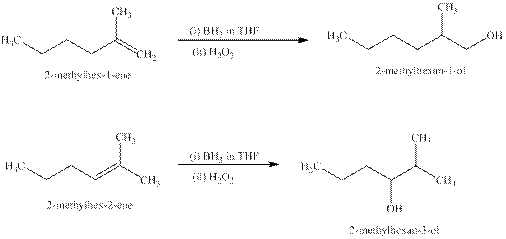
Explanation of Solution
Some reactions follow the rule of Markonikov’s addition but some do not.
Markonikov’s gave the rule purely on the basis of the observation of products received by him when performed the addition reaction on alkene.
Some reactions do not follow the rule because they follow the stability of the intermediate formed in the mechanism of that particular reaction.
The reaction of alkenes (
The products expected from the reaction of the products of part (b) and

Figure 6
The products expected from the reaction of the products of part (b) and
Want to see more full solutions like this?
Chapter 9 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- Understanding how substituents activate Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation HN NH2 Check X (Choose one) (Choose one) (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Aarrow_forwardIdentifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward* Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forward
- Draw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forwardPlease help me with the following questions for chemistry.arrow_forwardDraw the chemical structure [OR IUPAC name] of the following: a- m-chloromethoxybenzene b.arrow_forward
- Show by chemical equation the reaction of [HCN] and [CH3MgBr] with any alarrow_forwardGive the chemical equation for the preparation of: -Any aldehyde -Any keytonearrow_forward+ C8H16O2 (Fatty acid) + 11 02 → 8 CO2 a. Which of the above are the reactants? b. Which of the above are the products? H2o CO₂ c. Which reactant is the electron donor? Futty acid d. Which reactant is the electron acceptor? e. Which of the product is now reduced? f. Which of the products is now oxidized? 02 #20 102 8 H₂O g. Where was the carbon initially in this chemical reaction and where is it now that it is finished? 2 h. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forward
- → Acetyl-CoA + 3NAD+ + 1FAD + 1ADP 2CO2 + CoA + 3NADH + 1FADH2 + 1ATP a. Which of the above are the reactants? b. Which of the above are the products? c. Which reactant is the electron donor? d. Which reactants are the electron acceptors? e. Which of the products are now reduced? f. Which product is now oxidized? g. Which process was used to produce the ATP? h. Where was the energy initially in this chemical reaction and where is it now that it is finished? i. Where was the carbon initially in this chemical reaction and where is it now that it is finished? j. Where were the electrons initially in this chemical reaction and where is it now that it is finished?arrow_forwardRank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. OCH 3 (Choose one) OH (Choose one) Br (Choose one) Explanation Check NO2 (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Aarrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects O donating O withdrawing O no inductive effects Resonance Effects Overall Electron-Density ○ donating ○ withdrawing O no resonance effects O electron-rich O electron-deficient O similar to benzene Cl O donating O withdrawing ○ donating ○ withdrawing O no inductive effects O no resonance effects O Explanation Check O electron-rich O electron-deficient similar to benzene X © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





