
Concept explainers
(a)
Interpretation: The product formed by the treatment of given alcohol with
Concept introduction: The reaction of alcohols with halogen acids
Answer to Problem 9.39P
The products formed by the treatment of given alcohol with
Explanation of Solution
The given structure of alcohol is in the form of ball-and-stick model. It is converted into skeletal structure by replacing black ball with
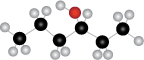
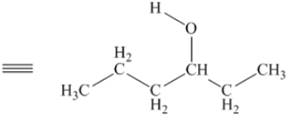
Figure 1
The above skeletal structure indicates that the given alcohol is secondary. It contains only one stereogenic center at
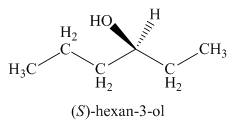
Figure 2
The reaction of alcohols with halogen acids
Since the given alcohol is secondary, it follows
The
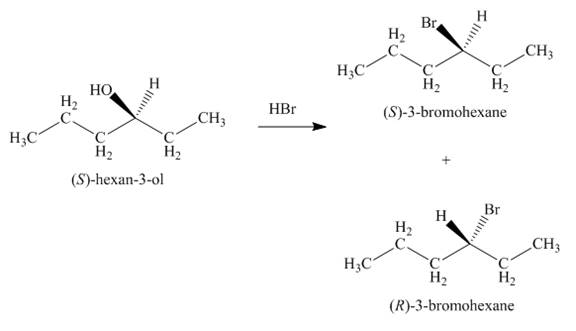
Figure 3
The products formed by the treatment of given alcohol with
(b)
Interpretation: The product formed by the treatment of given alcohol with
Concept introduction: Alkyl bromides are obtained by the reaction of
Answer to Problem 9.39P
The product formed by the treatment of given alcohol with
Explanation of Solution
Alkyl bromides are obtained by the reaction of
Since the given alcohol is secondary, it follows
The
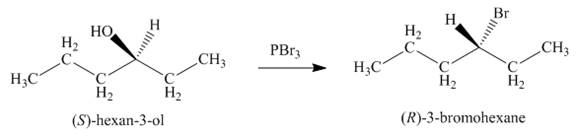
Figure 4
The product formed by the treatment of given alcohol with
(c)
Interpretation: The product formed by the treatment of given alcohol with
Concept introduction: The reaction of alcohols with halogen acids
Answer to Problem 9.39P
The products formed by the treatment of given alcohol with
Explanation of Solution
The reaction of alcohols with halogen acids
Since the given alcohol is secondary, it follows
The
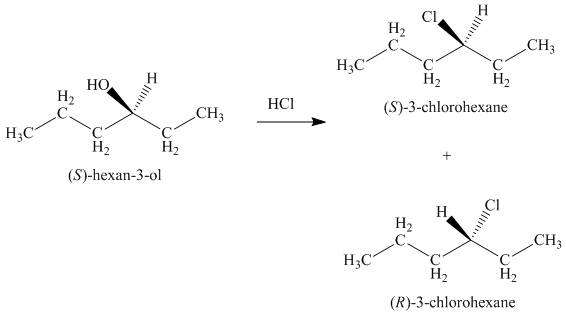
Figure 5
The products formed by the treatment of given alcohol with
(d)
Interpretation: The product formed by the treatment of given alcohol with
Concept introduction: Alkyl chlorides are obtained by the reaction of
Answer to Problem 9.39P
The product formed by the treatment of given alcohol with
Explanation of Solution
Alkyl chlorides are obtained by the reaction of
Since the given alcohol is secondary, it follows
The
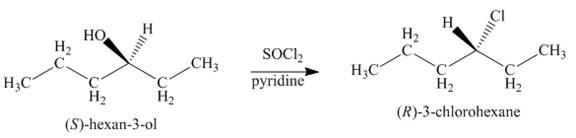
Figure 6
The product formed by the treatment of given alcohol with
Want to see more full solutions like this?
Chapter 9 Solutions
ORGANIC CHEMISTRY
- Please help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forwardPropose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forwardSelect the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward
- 4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forward
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
