
Concept explainers
(a)
Interpretation:
The condensed electron configurations and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(a)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
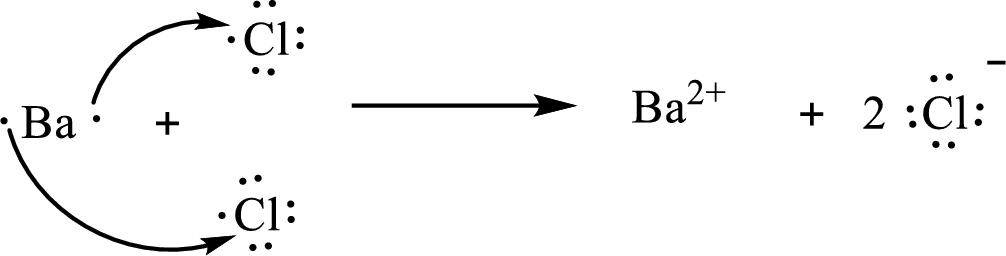
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a barium atom
The condensed electronic configuration of the chlorine atom
Barium atom loses two electrons to form
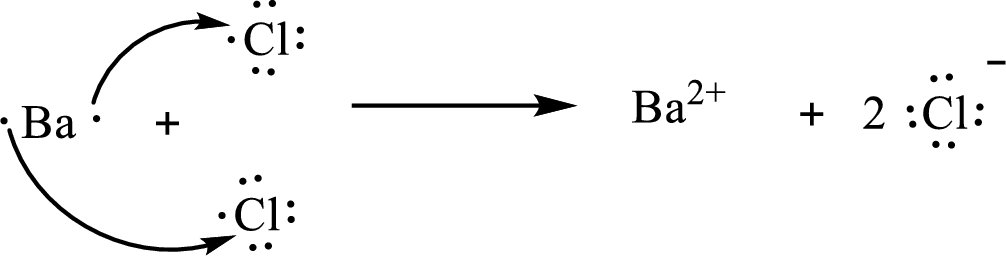
The condensed electronic configuration of
The condensed electronic configuration of
Barium atom loses two electrons to form
The Lewis orbital diagram is as follows:
(b)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(b)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
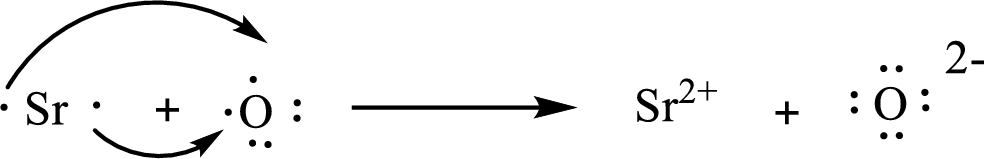
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a strontium atom
The condensed electronic configuration of oxygen atom
Strontium atom loses two electrons to form
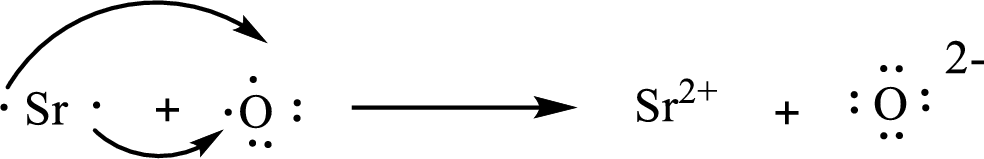
The condensed electronic configuration of
The condensed electronic configuration of
Strontium atom loses two electrons to form
The Lewis orbital diagram is as follows:
(c)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(c)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
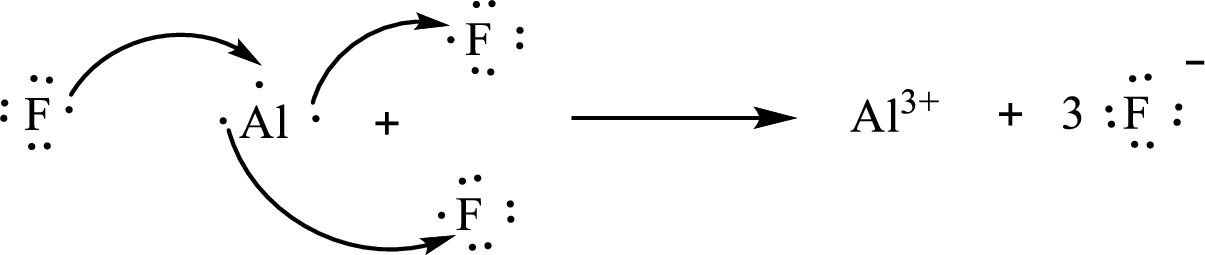
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of an aluminium atom
The condensed electronic configuration of the fluorine atom
Aluminium atom loses three electrons to form
The condensed electronic configuration of
The condensed electronic configuration of
Aluminium atom loses three electrons to form
The Lewis orbital diagram is as follows:
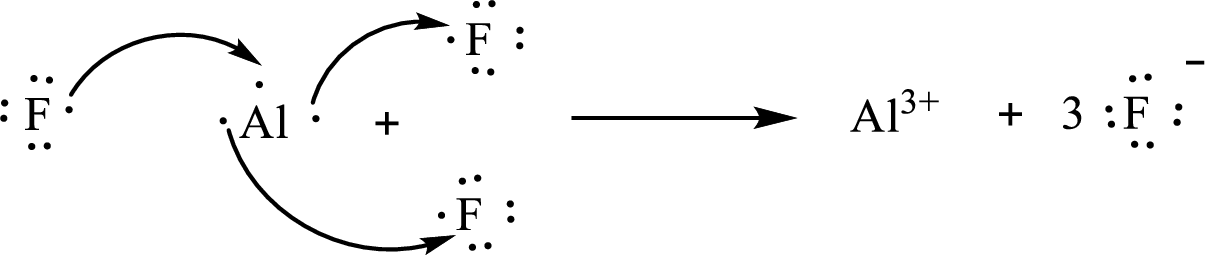
(d)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(d)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
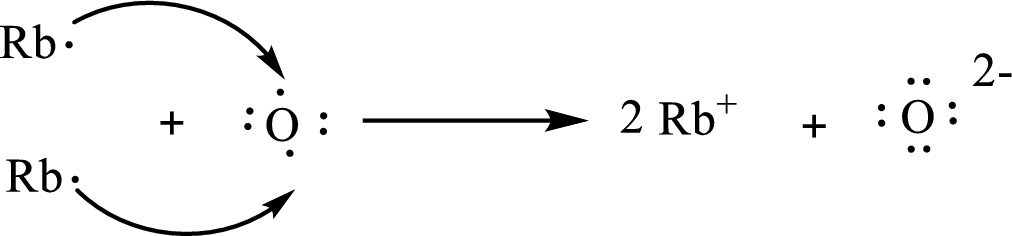
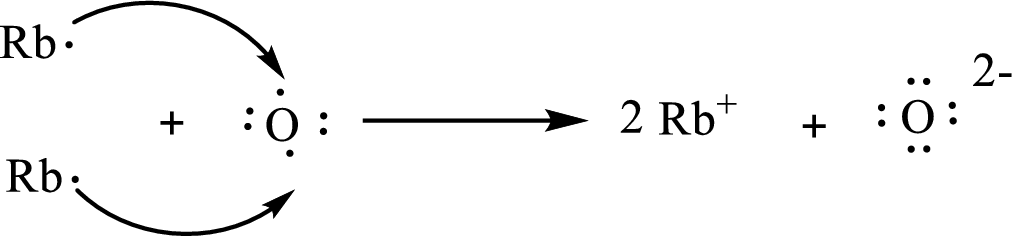
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a rubidium atom
The condensed electronic configuration of oxygen atom
Two rubidium atoms lose one electron respectively to form
The condensed electronic configuration of
The condensed electronic configuration of
Two rubidium atoms lose one electron respectively to form
The Lewis orbital diagram is as follows:
Want to see more full solutions like this?
Chapter 9 Solutions
Student Study Guide for Silberberg Chemistry: The Molecular Nature of Matter and Change
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





