
Concept explainers
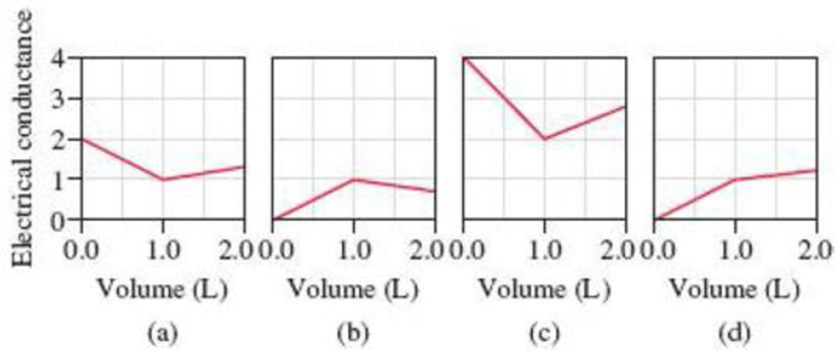
Because the acid-base and precipitation reactions discussed in this chapter all involve ionic species, their progress can be monitored by measuring the electrical conductance of the solution. Match each of the following reactions with one of the diagrams shown here. The electrical conductance is shown in arbitrary units. Explain the significance of the point at which the slope changes in each diagram.
(1) A 1.0 M KOH solution is added to 1.0 L of 1.0 M HC2H3O2.
(2) A 1.0 M NaOH solution is added to 1.0 L of 1.0 M HCl.
(3) A 1.0 M BaCl2 solution is added to 1.0 L of 1.0 M K2SO4.
(4) A 1.0 M NaCl solution is added to 1.0 L of 1.0 M AgNO3.
(5) A 1.0 M HC2H3O2 solution is added to 1.0 L of 1.0 M NH3.
(a)
Interpretation:
The given each reaction are should be matched with given each diagram and significance of slope change points in the given diagrams should be explained.
Concept introduction:
Precipitation reaction:
- If precipitate is formed, when two solutions are mixed together is called precipitation reaction.
- The amount of precipitate formed is related to the amount of reactants taken in to the reaction.
Neutralization reaction:
- The reaction between acid and base to gives a salt is the known as neutralization reaction.
Strong and weak electrolytes:
- The compound dissolved in water and completely dissociates to produces the ions is known as strong electrolytes.
- The compound dissolved in water but not completely dissociates to produces the ions is known as strong electrolytes.
Electrical conductivity of electrolytes:
- The strong electrolytes are having high electrical conductivity than weak electrolytes.
- The number of ion in solution is directly proportional to the electrical conductivity of electrolytes.
Conductivity titration:
- The measurement of electrical conductivity of titration mixture to gives a end point if the reaction.
- The sudden change in the slope is a equivalent point of the titration and it is the end point.
To find the electrical conductance, when
Answer to Problem 9.168QP
- The reactions (2) and (4) are matched with diagram (a).
- The reaction (5) is matched with diagram (b).
- The reaction (3) is matched with diagram (c).
- The reaction (1) is matched with diagram (d).
The slope change points in the given diagrams are end or equivalent points of the tractions.
Record the given data
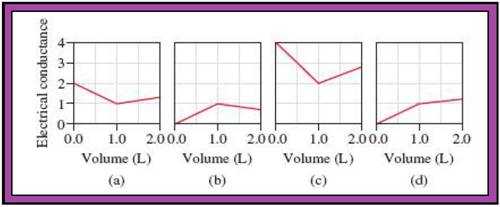
Fig.1
Explanation of Solution
If the conductance unit will be twice its concentration (molarity), when compound is completely dissociates into equal number of ions in solution.
Reaction of
Volume of
If
If
If Conductance unit of
(b)
Interpretation:
The given each reaction are should be matched with given each diagram and significance of slope change points in the given diagrams should be explained.
Concept introduction:
Precipitation reaction:
- If precipitate is formed, when two solutions are mixed together is called precipitation reaction.
- The amount of precipitate formed is related to the amount of reactants taken in to the reaction.
Neutralization reaction:
- The reaction between acid and base to gives a salt is the known as neutralization reaction.
Strong and weak electrolytes:
- The compound dissolved in water and completely dissociates to produces the ions is known as strong electrolytes.
- The compound dissolved in water but not completely dissociates to produces the ions is known as strong electrolytes.
Electrical conductivity of electrolytes:
- The strong electrolytes are having high electrical conductivity than weak electrolytes.
- The number of ion in solution is directly proportional to the electrical conductivity of electrolytes.
Conductivity titration:
- The measurement of electrical conductivity of titration mixture to gives a end point if the reaction.
- The sudden change in the slope is a equivalent point of the titration and it is the end point.
To find the electrical conductance when,
Answer to Problem 9.168QP
- The reactions (2) and (4) are matched with diagram (a).
- The reaction (5) is matched with diagram (b).
- The reaction (3) is matched with diagram (c).
- The reaction (1) is matched with diagram (d).
The slope change points in the given diagrams are end or equivalent points of the tractions.
Record the given data

Fig.1
Explanation of Solution
If the conductance unit will be twice its concentration (molarity), when compound is completely dissociates into equal number of ions in solution.
Reaction of
Volume of
If
If
(c)
Interpretation:
The given each reaction are should be matched with given each diagram and significance of slope change points in the given diagrams should be explained.
Concept introduction:
Precipitation reaction:
- If precipitate is formed, when two solutions are mixed together is called precipitation reaction.
- The amount of precipitate formed is related to the amount of reactants taken in to the reaction.
Neutralization reaction:
- The reaction between acid and base to gives a salt is the known as neutralization reaction.
Strong and weak electrolytes:
- The compound dissolved in water and completely dissociates to produces the ions is known as strong electrolytes.
- The compound dissolved in water but not completely dissociates to produces the ions is known as strong electrolytes.
Electrical conductivity of electrolytes:
- The strong electrolytes are having high electrical conductivity than weak electrolytes.
- The number of ion in solution is directly proportional to the electrical conductivity of electrolytes.
Conductivity titration:
- The measurement of electrical conductivity of titration mixture to gives a end point if the reaction.
- The sudden change in the slope is a equivalent point of the titration and it is the end point.
To find the electrical conductance when,
Answer to Problem 9.168QP
- The reactions (2) and (4) are matched with diagram (a).
- The reaction (5) is matched with diagram (b).
- The reaction (3) is matched with diagram (c).
- The reaction (1) is matched with diagram (d).
The slope change points in the given diagrams are end or equivalent points of the tractions.
Record the given data

Fig.1
Explanation of Solution
If the conductance unit will be twice its concentration (molarity), when compound is completely dissociates into equal number of ions in solution.
Reaction of
Volume of
If
If
(d)
Interpretation:
The given each reaction are should be matched with given each diagram and significance of slope change points in the given diagrams should be explained.
Concept introduction:
Precipitation reaction:
- If precipitate is formed, when two solutions are mixed together is called precipitation reaction.
- The amount of precipitate formed is related to the amount of reactants taken in to the reaction.
Neutralization reaction:
- The reaction between acid and base to gives a salt is the known as neutralization reaction.
Strong and weak electrolytes:
- The compound dissolved in water and completely dissociates to produces the ions is known as strong electrolytes.
- The compound dissolved in water but not completely dissociates to produces the ions is known as strong electrolytes.
Electrical conductivity of electrolytes:
- The strong electrolytes are having high electrical conductivity than weak electrolytes.
- The number of ion in solution is directly proportional to the electrical conductivity of electrolytes.
Conductivity titration:
- The measurement of electrical conductivity of titration mixture to gives a end point if the reaction.
- The sudden change in the slope is a equivalent point of the titration and it is the end point.
To find the electrical conductance, when
Answer to Problem 9.168QP
- The reactions (2) and (4) are matched with diagram (a).
- The reaction (5) is matched with diagram (b).
- The reaction (3) is matched with diagram (c).
- The reaction (1) is matched with diagram (d).
The slope change points in the given diagrams are end or equivalent points of the tractions.
Record the given data

Fig.1
Explanation of Solution
If the conductance unit will be twice its concentration (molarity), when compound is completely dissociates into equal number of ions in solution.
Reaction of
Volume of
If
If
(e)
Interpretation:
The given each reaction are should be matched with given each diagram and significance of slope change points in the given diagrams should be explained.
Concept introduction:
Precipitation reaction:
- If precipitate is formed, when two solutions are mixed together is called precipitation reaction.
- The amount of precipitate formed is related to the amount of reactants taken in to the reaction.
Neutralization reaction:
- The reaction between acid and base to gives a salt is the known as neutralization reaction.
Strong and weak electrolytes:
- The compound dissolved in water and completely dissociates to produces the ions is known as strong electrolytes.
- The compound dissolved in water but not completely dissociates to produces the ions is known as strong electrolytes.
Electrical conductivity of electrolytes:
- The strong electrolytes are having high electrical conductivity than weak electrolytes.
- The number of ion in solution is directly proportional to the electrical conductivity of electrolytes.
Conductivity titration:
- The measurement of electrical conductivity of titration mixture to gives a end point if the reaction.
- The sudden change in the slope is a equivalent point of the titration and it is the end point.
To find the electrical conductance, when
Answer to Problem 9.168QP
- The reactions (2) and (4) are matched with diagram (a).
- The reaction (5) is matched with diagram (b).
- The reaction (3) is matched with diagram (c).
- The reaction (1) is matched with diagram (d).
The slope change points in the given diagrams are end or equivalent points of the tractions.
Record the given data

Fig.1
Explanation of Solution
If the conductance unit will be twice its concentration (molarity), when compound is completely dissociates into equal number of ions in solution.
Reaction of
Volume of
If
If
Match the calculated conductance unit of each reaction in given diagrams in Fig.1.
- The reactions (2) and (4) are matched with diagram (a).
- The reaction (5) is matched with diagram (b).
- The reaction (3) is matched with diagram (c).
- The reaction (1) is matched with diagram (d).
The slope change points in the given diagrams are end or equivalent points of the tractions.
Want to see more full solutions like this?
Chapter 9 Solutions
CHEMISTRY:ATOMS FIRST (LL)>CUSTOM PKG.<
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forward
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





