
Bundle: Chemistry: The Molecular Science, 5th, Loose-Leaf + OWLv2 with Quick Prep 24-Months Printed Access Card
5th Edition
ISBN: 9781305367487
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 9, Problem 59QRT
Interpretation Introduction
Interpretation:
The atomic radius of xenon having fcc arrangement under given condition has to be determined.
Concept Introduction:
Relationship of radius with edge length:
A fcc unit cell can be represented as given below.
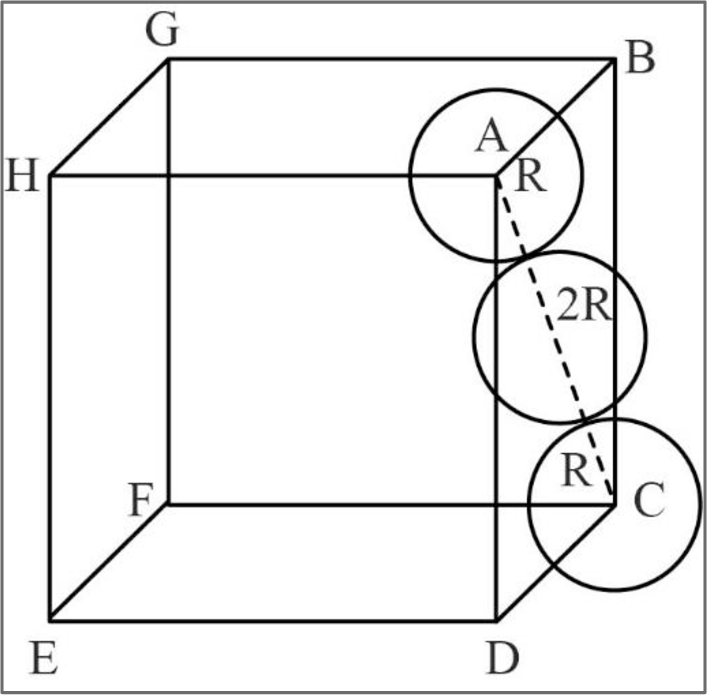
Figure 1
In fcc, the corner spheres are in touch with the face centered sphere as shown in the above figure. Hence, the face diagonal
Consider right angled triangle ACD.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
9. compore the Following two Venctions IN
termy Of Ronction Rate and explan in
detail the reasoning that led to your conclusion
+He p₁₂ 11-
ㅐ 15
.. +He
H #H
H
/
H
b. Compare
the Following too reactions 14
terms of reaction Rate and explain in detail
the reasoning that led to your conclusion
Н
d-C-
tłu
Na
+2446
е
-ll +2n
"H
a.
•Write all of the possible products
For the Following ronction
А
-----
H
-
H
H
+ H₂0 H+
Н
b. in Rite the complete reaction Mechaniszn
For the Formation of each product.
·C. Suggest what Reaction conditions could
Result in each product being the major
Product of the veaction:
a. Write the product For each of the
Following reactions
H
6-836-6
레
+H₂ N
A
H
A-C-C=C-C-CH + 2 Na +2 NH3 -
H H
b. Write the reaction Mechanism For.
reaction
each
Chapter 9 Solutions
Bundle: Chemistry: The Molecular Science, 5th, Loose-Leaf + OWLv2 with Quick Prep 24-Months Printed Access Card
Ch. 9.1 - Prob. 9.1CECh. 9.2 - Prob. 9.2CECh. 9.2 - Prob. 9.1PSPCh. 9.2 - What mass (g) of ethanol, CH3CH2OH(), can be...Ch. 9.3 - Prob. 9.3CECh. 9.3 - Prob. 9.4CECh. 9.3 - Prob. 9.3PSPCh. 9.4 - What types of solids are these substances? (a) The...Ch. 9.4 - Prob. 9.5PSPCh. 9.4 - Prob. 9.5E
Ch. 9.4 - Prob. 9.6CECh. 9.4 - Sublimation is an excellent means of purification...Ch. 9.4 - Prob. 9.6PSPCh. 9.4 - Prob. 9.8ECh. 9.4 - Prob. 9.9ECh. 9.5 - Predict which liquid—glycerol, HOCH2CH(OH)CH2OH,...Ch. 9.5 - Prob. 9.11CECh. 9.6 - Crystalline polonium has a primitive cubic unit...Ch. 9.6 - Calculate the unit cell edge length of copper...Ch. 9.6 - Vanadium metal crystallizes in a body-centered...Ch. 9.6 - Prob. 9.13ECh. 9.6 - Prob. 9.14ECh. 9.6 - Prob. 9.9PSPCh. 9.9 - Prob. 9.10PSPCh. 9.9 - The graph below is obtained when a liquid metal is...Ch. 9.9 - Look in Appendix D and compare the electron...Ch. 9.11 - Prob. 9.11PSPCh. 9 - Prob. ISPCh. 9 - Prob. IISPCh. 9 - Prob. IIISPCh. 9 - Prob. 1QRTCh. 9 - Prob. 2QRTCh. 9 - Prob. 3QRTCh. 9 - Prob. 4QRTCh. 9 - Prob. 5QRTCh. 9 - Prob. 6QRTCh. 9 - Which processes are endothermic? (a) Condensation...Ch. 9 - Prob. 8QRTCh. 9 - Prob. 9QRTCh. 9 - Prob. 10QRTCh. 9 - Prob. 11QRTCh. 9 - Prob. 12QRTCh. 9 - Prob. 13QRTCh. 9 - After exercising on a hot summer day and working...Ch. 9 - Prob. 15QRTCh. 9 - The molar vaporization enthalpy of methanol is...Ch. 9 - Prob. 17QRTCh. 9 - Mercury is highly toxic. Although it is a liquid...Ch. 9 - Prob. 19QRTCh. 9 - Prob. 20QRTCh. 9 - Prob. 21QRTCh. 9 - Prob. 22QRTCh. 9 - Prob. 23QRTCh. 9 - Prob. 24QRTCh. 9 - Prob. 25QRTCh. 9 - Prob. 26QRTCh. 9 - A liquid has a vapH of 38.7 kJ/mol and a boiling...Ch. 9 - Prob. 28QRTCh. 9 - The vapor pressure of ethanol, C2H5OH, at 50.0 C...Ch. 9 - Prob. 30QRTCh. 9 - Prob. 31QRTCh. 9 - Prob. 32QRTCh. 9 - Which would you expect to have the higher fusion...Ch. 9 - Prob. 34QRTCh. 9 - Prob. 35QRTCh. 9 - Prob. 36QRTCh. 9 - Prob. 37QRTCh. 9 - Prob. 38QRTCh. 9 - Prob. 39QRTCh. 9 - Prob. 40QRTCh. 9 - Prob. 41QRTCh. 9 - Prob. 42QRTCh. 9 - Prob. 43QRTCh. 9 - Prob. 44QRTCh. 9 - At the critical point for carbon dioxide, the...Ch. 9 - Prob. 46QRTCh. 9 - Prob. 47QRTCh. 9 - On the basis of the description given, classify...Ch. 9 - On the basis of the description given, classify...Ch. 9 - Prob. 50QRTCh. 9 - Prob. 51QRTCh. 9 - Prob. 52QRTCh. 9 - Prob. 53QRTCh. 9 - Prob. 54QRTCh. 9 - Prob. 55QRTCh. 9 - Prob. 56QRTCh. 9 - Prob. 57QRTCh. 9 - Prob. 58QRTCh. 9 - Prob. 59QRTCh. 9 - Prob. 60QRTCh. 9 - Prob. 61QRTCh. 9 - The ionic radii of Cs+ and Cl are 181 and 167 pm,...Ch. 9 - Prob. 63QRTCh. 9 - Prob. 64QRTCh. 9 - Prob. 65QRTCh. 9 - Tungsten has a body-centered cubic unit cell and...Ch. 9 - Prob. 67QRTCh. 9 - Prob. 68QRTCh. 9 - Prob. 69QRTCh. 9 - Prob. 70QRTCh. 9 - Prob. 71QRTCh. 9 - Prob. 72QRTCh. 9 - Prob. 73QRTCh. 9 - Prob. 74QRTCh. 9 - Prob. 75QRTCh. 9 - Prob. 76QRTCh. 9 - Prob. 77QRTCh. 9 - Prob. 78QRTCh. 9 - Prob. 79QRTCh. 9 - Prob. 80QRTCh. 9 - Which substance has the greatest electrical...Ch. 9 - Prob. 82QRTCh. 9 - Prob. 83QRTCh. 9 - Prob. 84QRTCh. 9 - Prob. 85QRTCh. 9 - Prob. 86QRTCh. 9 - What makes a glass different from a crystalline...Ch. 9 - Prob. 88QRTCh. 9 - Prob. 89QRTCh. 9 - Prob. 90QRTCh. 9 - Will a closed container of water at 70 C or an...Ch. 9 - Prob. 92QRTCh. 9 - Prob. 95QRTCh. 9 - Prob. 96QRTCh. 9 - Prob. 97QRTCh. 9 - Prob. 98QRTCh. 9 - Prob. 99QRTCh. 9 - Prob. 100QRTCh. 9 - Prob. 101QRTCh. 9 - Prob. 102QRTCh. 9 - Prob. 103QRTCh. 9 - Consider this information regarding two compounds....Ch. 9 - Prob. 105QRTCh. 9 - Prob. 106QRTCh. 9 - If you get boiling water at 100 C on your skin, it...Ch. 9 - Prob. 108QRTCh. 9 - The normal boiling point of SO2 is 263.1 K and...Ch. 9 - Butane is a gas at room temperature; however, if...Ch. 9 - Prob. 111QRTCh. 9 - Examine the nanoscale diagrams and the phase...Ch. 9 - Consider the phase diagram and heating-curve...Ch. 9 - Prob. 115QRTCh. 9 - Prob. 116QRTCh. 9 - The phase diagram for water over a relative narrow...Ch. 9 - Prob. 118QRTCh. 9 - Prob. 119QRTCh. 9 - Prob. 120QRTCh. 9 - Prob. 121QRTCh. 9 - Prob. 122QRTCh. 9 - Titanium metal crystallizes in a body-centered...Ch. 9 - Prob. 9.ACPCh. 9 - Prob. 9.BCPCh. 9 - Prob. 9.CCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=HCWwRh5CXYU;License: Standard YouTube License, CC-BY