
Why do we hybtidize atomic orbitals to explain the bonding in covalent compounds? What type of bonds form from hybrid orbitals: sigma or pi? Explain.
Interpretation: A reason corresponding to the hybridizing of atomic orbitals to explain the bonding in covalent compounds is to be stated. The type of bonds that are formed from hybrid orbitals is to be stated.
Concept introduction: Hybrid orbitals are formed by mixing of atomic orbitals when superimposed on each other in various proportions Hybrid orbitals having same energies are suitable for pairing of electrons which leads to the formation of chemical bond. Both sigma and pi bonds are formed from hybrid atomic orbital.
To determine: A reason corresponding to the hybridizing of atomic orbitals to explain the bonding in covalent compounds and the type of bonds that are formed from hybrid orbitals.
Answer to Problem 1RQ
Due to difference between the predicted structure and the experimental structure, it is rationalized that a new set of atomic orbitals is required which result from hybridization. If there is a “head to head” overlap of orbitals then sigma bond is formed and if there is a “side to side” overlap then pi bonds are formed.
Explanation of Solution
Hybrid orbitals are formed by mixing of atomic orbitals when superimposed on each other in various proportions. Hybridized orbitals are used in the formation of covalent compounds.
Due to discrepancy between the predicted structure and the experimental structure, a new set of atomic orbitals are used, commonly known as hybrid orbitals, which are required to explain the bonding structure.
During the formation of hybrid orbitals both type of bonds, sigma and pi, are formed.
Sigma bonds and pi bonds are formed by overlap of atomic orbitals.
Sigma bond is formed when there is a “head to head” overlap of orbitals and pi bond is formed when there is a “side to side” overlap.
Formation of sigma bond is shown in Figure 1.
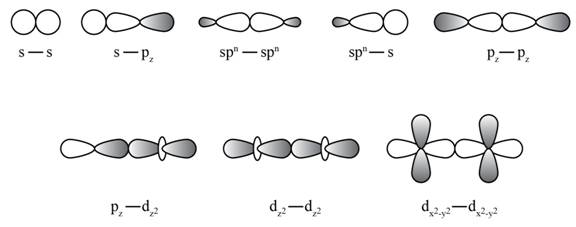
Figure 1
Formation of pi bond is shown in Figure 2.
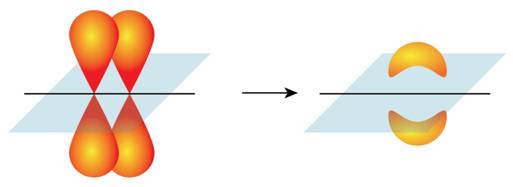
Figure 2
Hybrid orbitals are formed by mixing of atomic orbitals when superimposed on each other in various proportions.
During the formation of hybrid orbitals both type of bonds, sigma and pi, are formed
Want to see more full solutions like this?
Chapter 9 Solutions
EBK CHEMISTRY
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardTake a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forward
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward= 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forward
- Predict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe problem will not be graded for correctness, but you have to get a reasonable answer something that is either correct or very closer to the correct answer. The instructor professor wants us to do something that shows the answer but everything does not have to be correct. Ideally, yes, it has to be correct. Give it your best shot.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





