
Concept explainers
Interpretation:
A reactant that could be reacts with hexachlorocylopentadiene to gives chlordane through the Diels-Alder reaction has to be given.
Concept Introduction:
Cycloaddition reaction is a concerted addition reaction of two reactants to form a ring; in which two π-bonds are converted to two σ-bonds.
Diels-Alder reaction is a
The mechanism of the ring formation in the Diels-Alder reaction is drawn by using three arrows representing the shifting of π-bonds in the two π-systems in a clockwise or counter clockwise fashion, so that the ring formation will take place.
Cyclopentadiene can undergo Diels-Alder reaction itself to form a dimer,
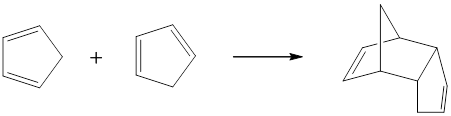
Endo-product is the major product in bicyclic-products of Diels-Alder reaction; because the electron-withdrawing substituents of dienophile and the newly forming π-bond of diene are interacted each other.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry (8th Edition)
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forwardLook at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forward
- Given 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- Concentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forwardExplain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forward
- Draw the line-angle formula of cis-2,3-dichloro-2-pentene. Then, draw the line-angle formula of trans-2,3-dichloro-2-pentene below. Draw the dash-wedge formula of cis-1,3-dimethylcyclohexane. Then, draw the dash-wedge formula of trans-1,3-dimethylcyclohexane below.arrow_forwardRecord the amounts measured and calculate the percent yield for Part 2 in the table below. Dicyclopentadiene measured in volume Cyclopentadiene measured in grams 0 Measured Calculated Mol Yield Mass (g) or Volume (mL) Mass (g) or Volume (ml) 0.6 2.955 Part 2 Measurements and Results Record the amounts measured and calculate the percent yield for Part 2 in the table below. 0.588 0.0044 2.868 0.0434 N/A Table view List view Measured Calculated Mol $ Yield Melting Point (C) Mass (g) or Volume (ml) Mass (g) or Volume (ml.) Cyclopentadiene 0.1 0.08 0.001189 measured in volume Maleic Anhydride 0.196 N/A cis-norbornene-5,6-endo- dicarboxylic anhydride 0.041 0.0002467 N/A N/A N/A 0.002 N/A N/A 128arrow_forwardDraw the condensed structural formula and line-angle formula for each: 2,3-dimethylheptane 3-bromo-2-pentanol 3-isopropyl-2-hexene 4-chlorobutanoic acidarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
