
EP ORGANIC CHEMISTRY,24 MONTH-OWLV2
9th Edition
ISBN: 9781305084391
Author: McMurry
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.SE, Problem 39MP
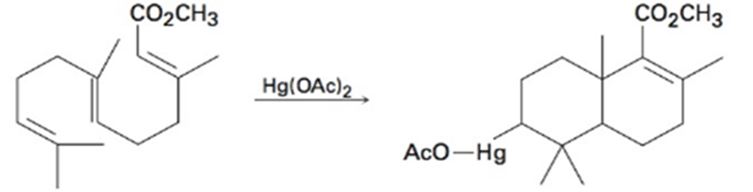
Use your general knowledge of
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
a. Explain Why electron withdrawing groups
tend to be meta-Directors. Your answer Should
lyclude all apropriate. Resonance contributing
Structures
fo. Explain why -ll is an outho -tura
drccton even though chlorine has a very High
Electronegativity
9. Write Me product as well as the reaction
Mechanism For each of the Following Vanctions
+H₂504
4.50+
T
C.
+212
Fellz
237
b. Praw the potential energy Diagrams For each
OF Mese Rauctions and account For any
differences that appear in the two potential
Puergy Diagrams
which of here two reactions 19 Found to be
Reversable, Rationalice your answer based upon
the venation mechanisms and the potential
energy diagrams.
9. Write Me product as well as the reaction
Mechanism For each of the Following Veritious
+H2504
4.50+
+ 1/₂ Felly
◎+
7
b. Praw he potential energy Diagrams For each
OF Mese Ronctions and account for any
differences that appeak in the two potential
Puergy Diagrams
Chapter 8 Solutions
EP ORGANIC CHEMISTRY,24 MONTH-OWLV2
Ch. 8.1 - Prob. 1PCh. 8.1 - How many alkene products, including E,Z isomers,...Ch. 8.2 - Prob. 3PCh. 8.2 - Addition of HCl to 1, 2-dimethylcyclohexene yields...Ch. 8.3 - Prob. 5PCh. 8.3 - Prob. 6PCh. 8.4 - Prob. 7PCh. 8.4 - From what alkenes might the following alcohols...Ch. 8.5 - Prob. 9PCh. 8.5 - What alkenes might be used to prepare the...
Ch. 8.5 - Tho following cycloalkene gives a mixture of two...Ch. 8.6 - Prob. 12PCh. 8.7 - Prob. 13PCh. 8.7 - Starting with an alkene, how would you prepare...Ch. 8.8 - Prob. 15PCh. 8.8 - Prob. 16PCh. 8.9 - What products would you expect from the following...Ch. 8.10 - Prob. 18PCh. 8.10 - Prob. 19PCh. 8.13 - Prob. 20PCh. 8.13 - What products are formed from hydration of...Ch. 8.SE - Name the following alkenes, and predict the...Ch. 8.SE - Prob. 23VCCh. 8.SE - Prob. 24VCCh. 8.SE - Prob. 25VCCh. 8.SE - Prob. 26MPCh. 8.SE - Prob. 27MPCh. 8.SE - Draw the structures of the organoboranes formed...Ch. 8.SE - Prob. 29MPCh. 8.SE - Provide the mechanism and products for the...Ch. 8.SE - Propose a curved-arrow mechanism to show how ozone...Ch. 8.SE - Prob. 32MPCh. 8.SE - Prob. 33MPCh. 8.SE - Prob. 34MPCh. 8.SE - 10-Bromo- α -chamigrene, a compound isolated from...Ch. 8.SE - Isolated from marine algae, prelaureatin is...Ch. 8.SE - Dichlorocarbene can be generated by heating sodium...Ch. 8.SE - Reaction of cyclohexene with mercury(II) acetate...Ch. 8.SE - Use your general knowledge of alkene chemistry to...Ch. 8.SE - Prob. 40MPCh. 8.SE - Hydroboration of 2-methyl-2-pentene at 25°C,...Ch. 8.SE - Prob. 42APCh. 8.SE - Suggest structures for alkenes that give the...Ch. 8.SE - Prob. 44APCh. 8.SE - Prob. 45APCh. 8.SE - Prob. 46APCh. 8.SE - Prob. 47APCh. 8.SE - Predict the products of the following reactions....Ch. 8.SE - Prob. 49APCh. 8.SE - How would you carry out the following...Ch. 8.SE - Draw the structure of an alkene that yields only...Ch. 8.SE - Show the structures of alkenes that give the...Ch. 8.SE - Prob. 53APCh. 8.SE - Which of the following alcohols could not be made...Ch. 8.SE - Prob. 55APCh. 8.SE - Prob. 56APCh. 8.SE - Prob. 57APCh. 8.SE - Compound A has the formula C10HI6. On catalytic...Ch. 8.SE - Prob. 59APCh. 8.SE - Prob. 60APCh. 8.SE - Prob. 61APCh. 8.SE - Draw the structure of a hydrocarbon that absorbs 2...Ch. 8.SE - Prob. 63APCh. 8.SE - The sex attractant of the common housefly is a...Ch. 8.SE - Prob. 65APCh. 8.SE - Prob. 66APCh. 8.SE - α-Terpinene, C10H16, is a pleasant-smelling...Ch. 8.SE - Prob. 68APCh. 8.SE - Prob. 69APCh. 8.SE - Prob. 70APCh. 8.SE - Prob. 71APCh. 8.SE - Prob. 72AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forwardGiven the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forward
- Electrochemistry. State the difference between E and E0.arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forward
- Calculate the free energy of formation of 1 mol of Cu in cells where the electrolyte is 1 mol dm-3 Cu2+ in sulfate solution, pH 0. E° for the Cu2+/Cu pair in this medium is +142 mV versus ENH.Assume the anodic reaction is oxygen evolution.Data: EH2 = -0.059 pH (V) and EO2 = 1.230 - 0.059 pH (V); 2.3RT/F = 0.059 Varrow_forwardIf the normal potential for the Fe(III)/Fe(II) pair in acid at zero pH is 524 mV Hg/Hg2Cl2 . The potential of the saturated calomel reference electrode is +246 mV versus the NHE. Calculate E0 vs NHE.arrow_forwardGiven the galvanic cell whose scheme is: (-) Zn/Zn2+ ⋮⋮ Ag+/Ag (+). If we know the normal potentials E°(Zn2+/Zn) = -0.76V and E°(Ag+/Ag) = 0.799 V. Indicate the electrodes that are the anode and the cathode and calculate the E0battery.arrow_forward
- Indicate the functions that salt bridges have in batteries.arrow_forwardIn the battery:Pt | H2 (g) | H+ (aq) | Fe2+ (aq) | FeIndicate the cathode and anode.arrow_forwardWrite the equations that occur when the electrode Pb (s) | PbI2 (s) | KI (ac) in a galvanic cell. a) It functions as a positive electrode b) It functions as a negative electrode c) What is the ion with respect to which this electrode is reversible?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License