
Concept explainers
Name the following
a)
Interpretation:
The name of the alkene shown to be given. The products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid, are to be given.
Concept introduction:
When alkenes react with meta-chloroperoxybenzoic acid, oxygen atom adds to the double bond to give epoxides.
Upon treatment with KMnO4 in aqueous acid, if the alkene the double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bond is cleaved and each carbon in the double bond gets attached to an oxygen atom to give carbonyl compounds as products. If in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to an aldehyde.
To give:
The name of the alkene shown and the products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid.
Answer to Problem 22VC
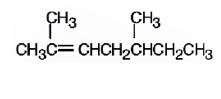
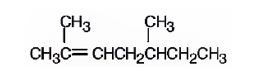
The name of the alkene shown is 2,5-dimethyl-2-heptene.
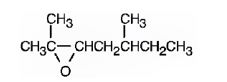
The product formed when it reacts with meta-chloroperoxybenzoic acid is
The products formed when it reacts with KMnO4 in aqueous acid are
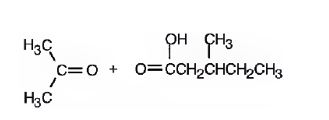
The products formed when it reacts with O3, followed by Zn in acetic acid are
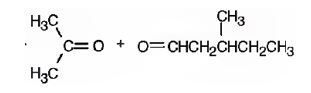
Explanation of Solution
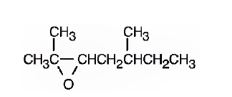
2,5-dimethyl-2-heptene has a double bond between C2&C3. When treated with meta-chloroperoxybenzoic acid, oxygen atom adds to both C2&C3 to give an epoxide.
In 2,5-dimethyl-2-heptene, C2 has no hydrogens attached. So it is oxidized to a ketone.C3 has one hydrogen and is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bond between C2&C3 in 2,5-dimethyl-2-heptene is cleaved and each carbon gets attached to an oxygen atom to give a ketone and aldehyde as products.
The name of the alkene shown is 2,5-dimethyl-2-heptene is
The product formed when it reacts with meta-chloroperoxybenzoic acid is
The products formed when it reacts with KMnO4 in aqueous acid are
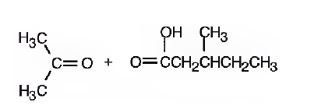
The products formed when it reacts with O3, followed by Zn in acetic acid are
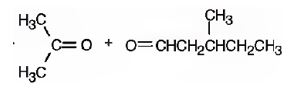
b)
Interpretation:
The name of the alkene shown to be given. The products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid, are to be given.
Concept introduction:
When alkenes react with meta-chloroperoxybenzoic acid, oxygen atom adds to the double bond to give epoxides.
Upon treatment with KMnO4 in aqueous acid, if in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bonded is cleaved and each carbon in the double bond gets attached to an oxygen atom to give carbonyl compounds as products. If in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to an aldehyde.
To give:
The name of the alkene shown and the products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid.
Answer to Problem 22VC
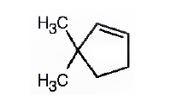
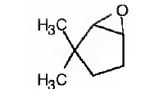
The name of the alkene shown is 3,3-dimethylcyclopentene.
The product formed when it reacts with meta-chloroperoxybenzoic acid is
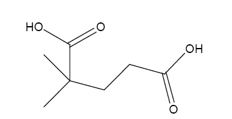
The products formed when it reacts with KMnO4 in aqueous acid are
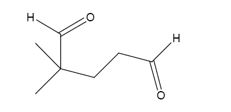
The products formed when it reacts with O3, followed by Zn in acetic acid are
Explanation of Solution
3,3-dimethylcyclopentene has a double bond between C1&C2. When treated with meta-chloroperoxybenzoic acid, oxygen atom adds to both C1&C2 to give an epoxide.
In 3,3-dimethylcyclopentene both C1&C2 have one hydrogen attached to them. So they are oxidized to carboxylic acids when treated with KMnO4.
Upon treatment with O3, followed by Zn in acetic acid the double bond between C1&C2 in 3,3-dimethylcyclopentene is cleaved and each carbon with one hydrogen gets attached to an oxygen atom to yield a dialdehyde as product.
The name of the alkene shown is 3,3-dimethylcyclopentene.

The products formed when it reacts with meta-chloroperoxybenzoic acid are

The products formed when it reacts with KMnO4 in aqueous acid are

The products formed when it reacts with O3, followed by Zn in acetic acid are

Want to see more full solutions like this?
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forwardThe following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forward
- The following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forwardIf we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forwardBriefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forward
- Explain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0 262.7 QUESTION: For both groups of data provide answers to the calculations attached in the imagearrow_forward
- 7. Concentration and uncertainty in the estimate of concentration (class data) Class mean for sample (Regular) |[Cl-] (mmol/L) class mean Sn za/2 95% Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Confidence Interval (mg/100 mL)arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardGive reason(s) for six from the followings [using equations if possible] a. Addition of sodium carbonate to sulfanilic acid in the Methyl Orange preparation. b. What happened if the diazotization reaction gets warmed up by mistake. c. Addition of sodium nitrite in acidified solution in MO preparation through the diazotization d. Using sodium dithionite dihydrate in the second step for Luminol preparation. e. In nitroaniline preparation, addition of the acid mixture (nitric acid and sulfuric acid) to the product of step I. f. What is the main reason of the acylation step in nitroaniline preparation g. Heating under reflux. h. Fusion of an organic compound with sodium. HAND WRITTEN PLEASEarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

