
Concept explainers
(a)
Interpretation: The mechanism for the given chemical transformation is to be interpreted.
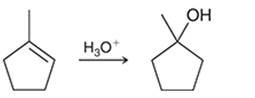
Concept introduction:
(b)
Interpretation: The mechanism for the given chemical transformation is to be interpreted.
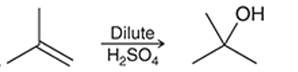
Concept introduction:
Alkenes are unsaturated hydrocarbons with at least one double bond between the carbon atoms. The acid-catalyzed hydration reactions of alkene involve the reaction of an alkene with
(c)
Interpretation: The mechanism for the given chemical transformation is to be interpreted.
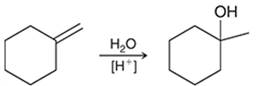
Concept introduction:
Alkenes are unsaturated hydrocarbons with at least one double bond between the carbon atoms. The acid-catalyzed hydration reactions of alkene involve the reaction of an alkene with
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardDraw a Newman projection for the molecule below from the perspective indicated. Which of the groups (letters A-H) are methyl groups? CH3 H H H A H B ☑ >> H. ABCDEFG I H -H CH3 G D CH F E Numeric 4 points How many gauche interactions exist in the conformation shown in the previous problem? 1arrow_forward
- HELP NOW PLEASE ! ASAP! URGENT!arrow_forwardHELP NOW PLEASE ! ASAP! URGENT!arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward
- Pls help.arrow_forward13) When solid barium phosphate is in equilibrium with its ions, the ratio of barium ions to phosphate ions would be: a. 1:1 b. 2:3 c. 3:2 d. 2:1 14) The pH of a 0.05 M solution of HCl(aq) at 25°C is 15) The pH of a 0.20 M solution of KOH at 25°C isarrow_forwardPls help.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





