
(a)
Interpretation:
The configuration of the substitution products formed from the given reaction has to be drawn.
Concept Introduction:
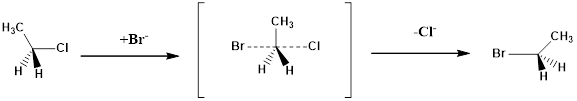
Structure of the substrate plays a major role in
Solvolysis: The reaction is defined as solvolysis reaction if solvent acts as nucleophile in the given reaction.
Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
(b)
Interpretation:
The configuration of the substitution products formed from the given reaction has to be drawn.
Concept Introduction:
Primary carbocation < secondary carbocation < tertiary carbocation
Transition State: The state which defines the highest potential energy with respect to reaction co-ordinate between reactant and product. It is usually denoted by using the symbol ‘≠’.
Nucleophile: donates pair of electrons to positively charged substrate resulting in the formation of
Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
Assign numbering to the groups which are bonded to the chiral carbon based on the molecular weight and electronegativity.
If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
EBK ESSENTIAL ORGANIC CHEMISTRY
- Can I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole



