
Interpretation:
How the rate of the reaction between an
Concept Introduction:
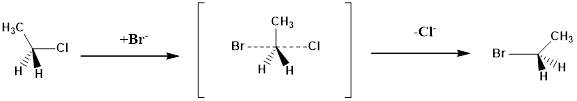
Structure of the substrate plays a major role in
Solvent: it is substance which dissolves the chemical substrate. They are classified as polar protic and
Polar parotic solvent: It contains at least one
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Essential Organic Chemistry Study Guide & Solution Manual, Books a la Carte Edition
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- At 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardPredict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


