
GENERAL,ORGANIC,+BIOCHEM.(LL) >CUSTOM<
10th Edition
ISBN: 9781264116546
Author: Denniston
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8.1, Problem 8.2PP
(a)
Interpretation Introduction
Interpretation:
The conjugate base of given acids has to be written. Also, the stronger acid has to be identified for each pair.
Concept Introduction:
Acids and bases classified as strong when the reaction with water undergoes 100% completion and as weak when the reaction with water is much less than 100% complete. The relative strength of Acid-base are given by
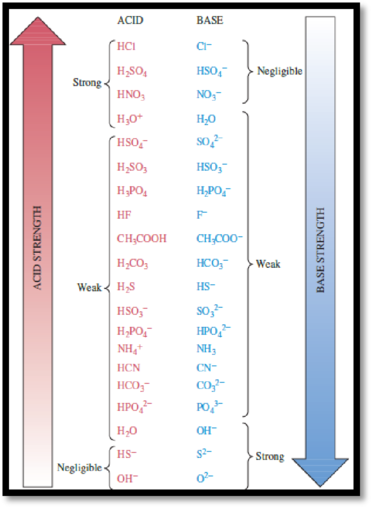
Figure 1
(b)
Interpretation Introduction
Interpretation:
The conjugate acid of given bases has to be written. Also, the stronger base has to be identified.
Concept Introduction:
Refer to part (a)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
These are in the wrong boxes. Why does the one on the left have a lower molar mass than the one on the right?
SYNTHESIS REACTIONS. For the following reactions, synthesize the given products from the given reactants.
Multiple reactions/steps will be needed. For the one of the steps (ie reactions) in each synthesis, write out the
mechanism for that reaction and draw an energy diagram showing the correct number of hills and valleys for
that step's mechanism.
CI
b.
a.
Use acetylene (ethyne)
and any alkyl halide as
your starting materials
Br
C.
d.
"OH
OH
III.
OH
Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely:
(a) 0.200 M HCl
Chapter 8 Solutions
GENERAL,ORGANIC,+BIOCHEM.(LL) >CUSTOM<
Ch. 8.1 - Classify CH3COO− as a Brønsted-Lowry acid or base,...Ch. 8.1 - Prob. 8.1QCh. 8.1 - Prob. 8.2QCh. 8.1 - Write an equation for the reversible reactions of...Ch. 8.1 - Prob. 8.4QCh. 8.1 - Prob. 8.5QCh. 8.1 - Prob. 8.6QCh. 8.1 - Prob. 8.2PPCh. 8.1 - Analysis of a patient’s blood sample indicated...Ch. 8.1 - Prob. 8.7Q
Ch. 8.1 - The hydroxide ion concentration in a sample of...Ch. 8.2 - Calculate the pH of a 1.0 × 10−4 M solution of...Ch. 8.2 - Calculate the [H3O+] of a solution of HNO3 that...Ch. 8.2 - Calculate the pH corresponding to a 1.0 × 10−2 M...Ch. 8.2 - Calculate the [H3O+] and [OH−] of a potassium...Ch. 8.2 - Calculate the [H3O+] corresponding to pH =...Ch. 8.2 - Prob. 8.9PPCh. 8.2 - Calculate the [OH–] of a 1.0 × 10–3 M solution of...Ch. 8.2 - Prob. 8.10QCh. 8.3 - Calculate the molar concentration of a sodium...Ch. 8.4 - A buffer solution is prepared in such a way that...Ch. 8.4 - Prob. 8.12PPCh. 8.4 - Prob. 8.11QCh. 8.4 - Prob. 8.12QCh. 8.4 - Prob. 8.13QCh. 8.4 - Prob. 8.14QCh. 8.4 - Prob. 8.15QCh. 8.4 - Prob. 8.16QCh. 8.4 - Prob. 8.17QCh. 8.4 - Explain how the pH of blood would change under...Ch. 8.4 - Write the Henderson-Hasselbalch expression for the...Ch. 8.4 - Prob. 8.20QCh. 8.5 - Prob. 8.21QCh. 8.5 - Prob. 8.22QCh. 8.5 - Prob. 8.23QCh. 8.5 - Prob. 8.24QCh. 8.5 - Chrome plating involves the reduction of Cr3+(aq)...Ch. 8.5 - Prob. 8.26QCh. 8 - Prob. 8.27QPCh. 8 - Define a base according to the Arrhenius...Ch. 8 - What are the essential differences between the...Ch. 8 - Why is ammonia described as a Brønsted-Lowry base...Ch. 8 - Classify each of the following as either a...Ch. 8 - Classify each of the following as either a...Ch. 8 - Classify each of the following as either a...Ch. 8 - Classify each of the following as either a...Ch. 8 - Write an equation for the reaction of each of the...Ch. 8 - Write an equation for the reaction of each of the...Ch. 8 - Write the formula of the conjugate acid of CN−.
Ch. 8 - Write the formula of the conjugate acid of Br−.
Ch. 8 - Write the formula of the conjugate base of HI.
Ch. 8 - Write the formula of the conjugate base of HCOOH.
Ch. 8 - Write the formula of the conjugate acid of NO3−.
Ch. 8 - Write the formula of the conjugate acid of F−.
Ch. 8 - Which is the stronger base, NO3− or CN−?
Ch. 8 - Prob. 8.44QPCh. 8 - Prob. 8.45QPCh. 8 - Which is the stronger base, F− or CH3COO−?
Ch. 8 - Identify the conjugate acid-base pairs in each of...Ch. 8 - Identify the conjugate acid-base pairs in each of...Ch. 8 - Distinguish between the terms acid-base strength...Ch. 8 - Label each of the following as a strong or weak...Ch. 8 - Label each of the following as a strong or weak...Ch. 8 - Calculate the [H3O+] of an aqueous solution that...Ch. 8 - Calculate the [H3O+] of an aqueous solution that...Ch. 8 - Calculate the [OH−] of an aqueous solution that...Ch. 8 - Prob. 8.56QPCh. 8 - Prob. 8.57QPCh. 8 - What is the concentration of hydronium ions in an...Ch. 8 - Prob. 8.59QPCh. 8 - Consider two beakers, one containing 0.10 M NaOH...Ch. 8 - Calculate the pH of a solution that is:
1.0 × 10−2...Ch. 8 - Calculate the pH of a solution that is:
1.0 × 10−1...Ch. 8 - Calculate [H3O+] for a solution of nitric acid for...Ch. 8 - Calculate [H3O+] for a solution of hydrochloric...Ch. 8 - Prob. 8.65QPCh. 8 - Prob. 8.66QPCh. 8 - Calculate both [H3O+] and [OH−] for a solution for...Ch. 8 - Calculate both [H3O+] and [OH−] for a solution for...Ch. 8 - What is a neutralization reaction?
Ch. 8 - Describe the purpose of a titration.
Ch. 8 - Prob. 8.71QPCh. 8 - The pH of urine may vary between 4.5 and 8.2....Ch. 8 - Criticize the following statement: A lakewater...Ch. 8 - Can a dilute solution of a strong acid ever have a...Ch. 8 - What is the H3O+ concentration of a solution with...Ch. 8 - What is the H3O+ concentration of a solution with...Ch. 8 - Prob. 8.77QPCh. 8 - Prob. 8.78QPCh. 8 - Calculate the pH of a solution that has [H3O+] =...Ch. 8 - Calculate the pH of a solution that has [H3O+] =...Ch. 8 - Calculate the pH of a solution that has [OH−] =...Ch. 8 - Calculate the pH of a solution that has [OH−] =...Ch. 8 - Prob. 8.83QPCh. 8 - Prob. 8.84QPCh. 8 - Prob. 8.85QPCh. 8 - Prob. 8.86QPCh. 8 - Write an equation to represent the neutralization...Ch. 8 - Write an equation to represent the neutralization...Ch. 8 - Prob. 8.89QPCh. 8 - Prob. 8.90QPCh. 8 - Prob. 8.91QPCh. 8 - Prob. 8.92QPCh. 8 - Titration of 15.00 mL of HCl solution requires...Ch. 8 - Titration of 17.85 mL of HNO3 solution requires...Ch. 8 - Prob. 8.95QPCh. 8 - Prob. 8.96QPCh. 8 - Prob. 8.97QPCh. 8 - Prob. 8.98QPCh. 8 - Which of the following are capable of forming a...Ch. 8 - Which of the following are capable of forming a...Ch. 8 - Prob. 8.101QPCh. 8 - Prob. 8.102QPCh. 8 - Prob. 8.103QPCh. 8 - Prob. 8.104QPCh. 8 - For the equilibrium situation involving acetic...Ch. 8 - Prob. 8.106QPCh. 8 - Prob. 8.107QPCh. 8 - Prob. 8.108QPCh. 8 - Prob. 8.109QPCh. 8 - For the buffer system described in Question 8.105,...Ch. 8 - Prob. 8.111QPCh. 8 - Prob. 8.112QPCh. 8 - Prob. 8.113QPCh. 8 - Prob. 8.114QPCh. 8 - Prob. 8.115QPCh. 8 - Prob. 8.116QPCh. 8 - Prob. 8.117QPCh. 8 - Prob. 8.118QPCh. 8 - In the following reaction, identify the oxidized...Ch. 8 - Prob. 8.120QPCh. 8 - Prob. 8.121QPCh. 8 - Prob. 8.122QPCh. 8 - Prob. 8.123QPCh. 8 - Prob. 8.124QPCh. 8 - Prob. 8.125QPCh. 8 - Prob. 8.126QPCh. 8 - Prob. 1MCPCh. 8 - Prob. 2MCPCh. 8 - Prob. 3MCPCh. 8 - Prob. 4MCPCh. 8 - Prob. 5MCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY