
(a)
Interpretation:
The mechanism for the given elimination reaction including carbocation rearrangement is to be drawn.
Concept introduction:
The
Answer to Problem 8.65P
The
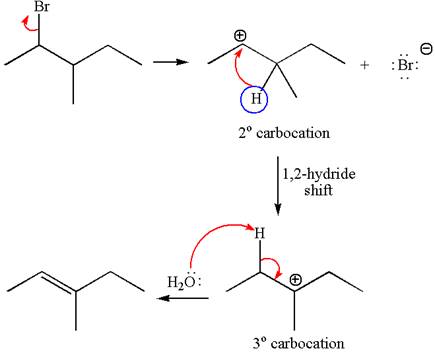
Explanation of Solution
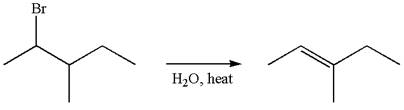
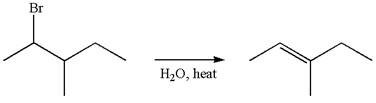
The given reaction equation is
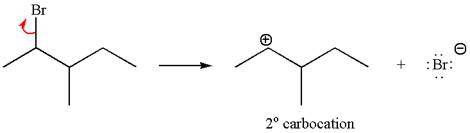
In first step, the leaving group
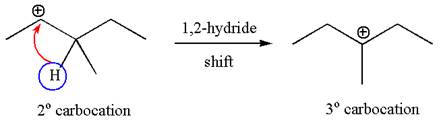
The carbocation formed is rearranged by
The water molecule acts as a base and abstracts a proton from the carbon adjacent to the carbocation, forming
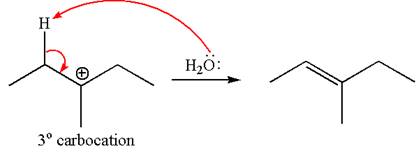
The mechanism for the given elimination reaction is drawn to show the carbocation rearrangement by
(b)
Interpretation:
The mechanism for the given elimination reaction without carbocation rearrangement is to be drawn.
Concept introduction:
The
Answer to Problem 8.65P
The
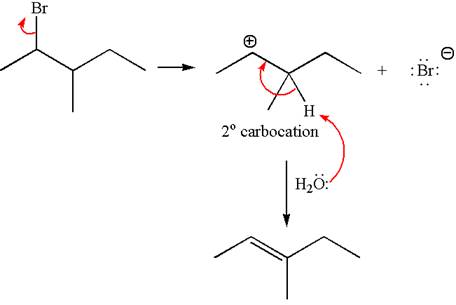
Explanation of Solution
The given reaction equation is
In the first step, the leaving group
In the second step, without rearrangement, the proton is eliminated by the base
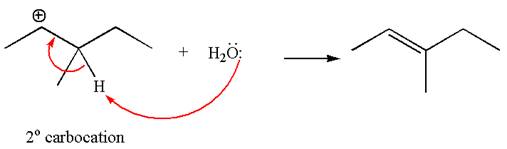
The mechanism for the given elimination reaction is drawn to without rearrangement step, indicating that the same product is formed with or without rearrangement.
(c)
Interpretation:
It is to be explained how the
Concept introduction:
The
Answer to Problem 8.65P
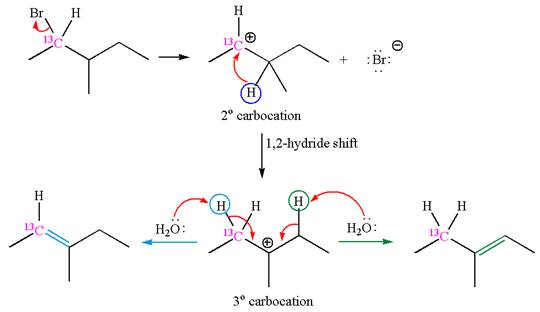
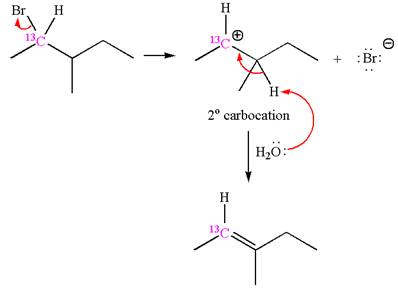
The reaction with carbocation rearrangement gave two products, and the reaction without carbocation rearrangement gave only one product, as shown below, indicating that the
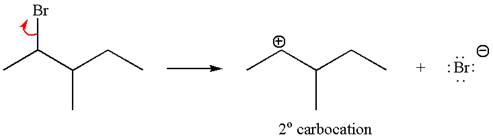
Reaction with rearrangement:
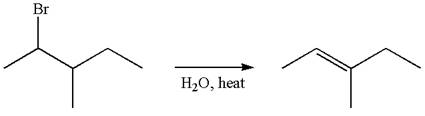
Reaction without rearrangement:
Explanation of Solution
The given reaction equation is
If the carbon bonded to the leaving group in the given substrate is labelled as
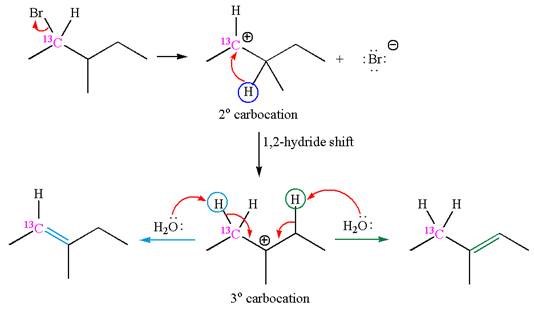
In one product, one of the double bonded carbon is
If the reaction proceeds without rearrangement, then only one product is formed where one of the double bonded carbon is
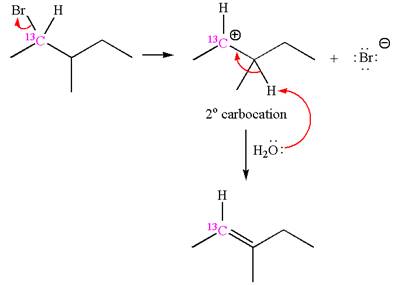
As the reaction with rearrangement of carbocation formed two products, and reaction without rearrangement formed only one product, it indicates that the E1 products depend on whether the rearrangement occurred.
It is explained that the E1 products depend on whether the reaction includes carbocation rearrangement occurring with
(d)
Interpretation:
How the deuterium isotope labeling is useful to determine whether the rearrangement is occurred in given
Concept introduction:
The
Answer to Problem 8.65P
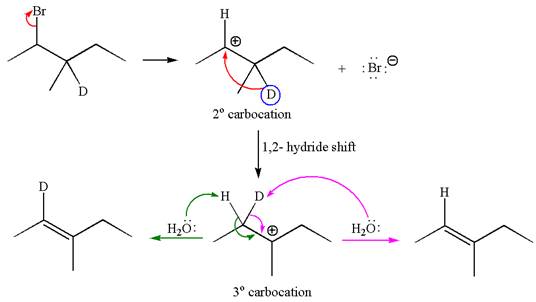
The reaction with carbocation rearrangement by migration of deuterium gave two products, and the reaction without carbocation rearrangement gave only one product as shown below, indicating that the deuterium isotope labeling is useful to determine whether the rearrangement occurred in the given
Reaction with rearrangement:
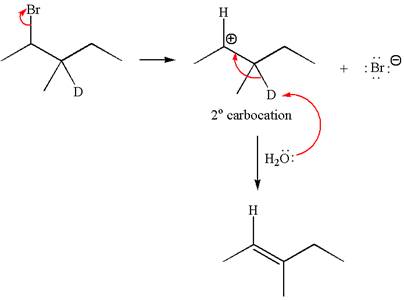
Reaction without rearrangement:
Explanation of Solution
The given reaction equation is
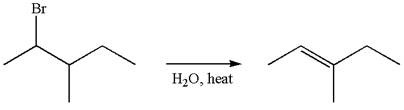
If the migrating hydrogen in the given substrate is replaced with deuterium, then two products are formed when the reaction occurred through carbocation rearrangement. The detailed mechanism is as follows:
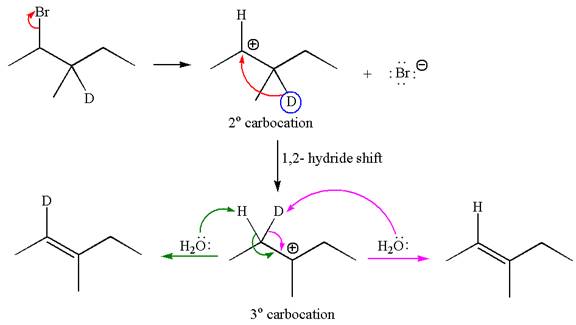
One product is formed by elimination of hydrogen atom and another by elimination of deuterium atom.
If the reaction proceeds without rearrangement, only one product is formed by elimination of deuterium atom. The detailed mechanism is as follows:
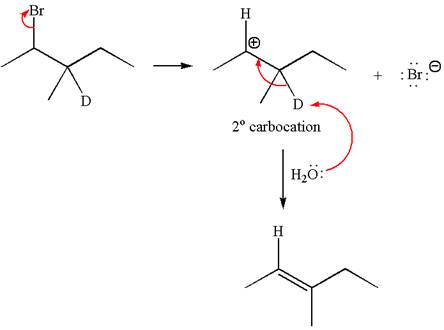
As the reaction with rearrangement of carbocation formed two products and reaction without rearrangement formed only one product, it indicates the E1 products depend on whether the rearrangement occurs.
It is explained on the basis of formation of different products that deuterium isotope labeling is useful to determine whether the rearrangement occurred in the given
Want to see more full solutions like this?
Chapter 8 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- For the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward
- :0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forwardPlease help me with this the problem is so confusingarrow_forward
- 14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forwardanswer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
