
(a)
Interpretation:
The mechanism for the given elimination reaction including carbocation rearrangement is to be drawn.
Concept introduction:
The
Answer to Problem 8.65P
The
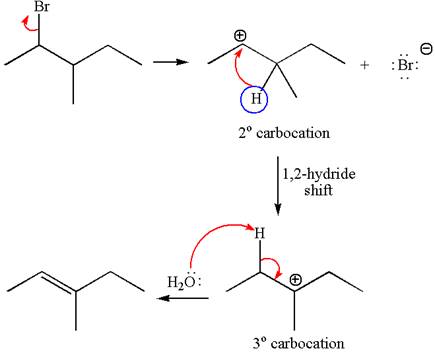
Explanation of Solution
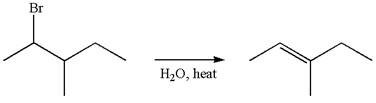
The given reaction equation is
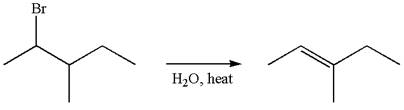
In first step, the leaving group
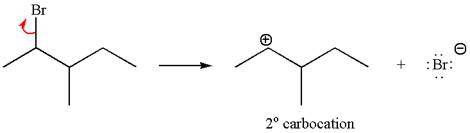
The carbocation formed is rearranged by
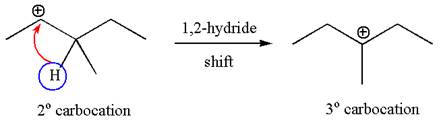
The water molecule acts as a base and abstracts a proton from the carbon adjacent to the carbocation, forming
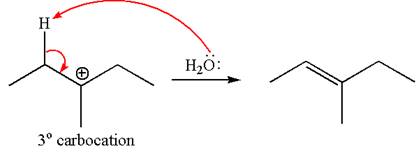
The mechanism for the given elimination reaction is drawn to show the carbocation rearrangement by
(b)
Interpretation:
The mechanism for the given elimination reaction without carbocation rearrangement is to be drawn.
Concept introduction:
The
Answer to Problem 8.65P
The
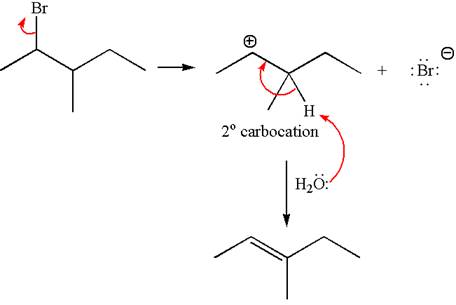
Explanation of Solution
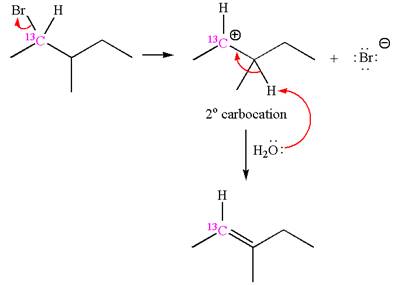
The given reaction equation is
In the first step, the leaving group
In the second step, without rearrangement, the proton is eliminated by the base
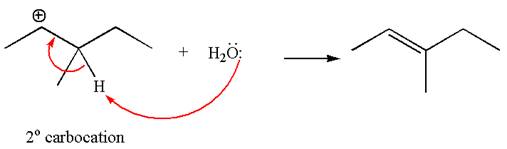
The mechanism for the given elimination reaction is drawn to without rearrangement step, indicating that the same product is formed with or without rearrangement.
(c)
Interpretation:
It is to be explained how the
Concept introduction:
The
Answer to Problem 8.65P
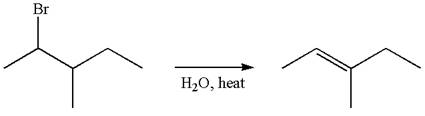
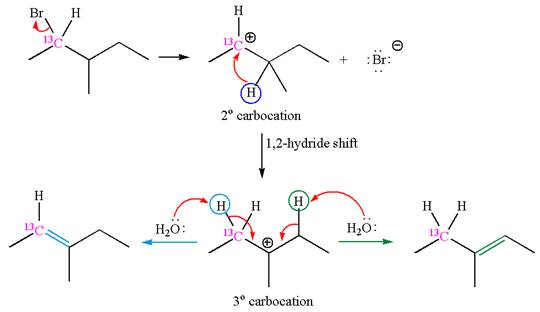
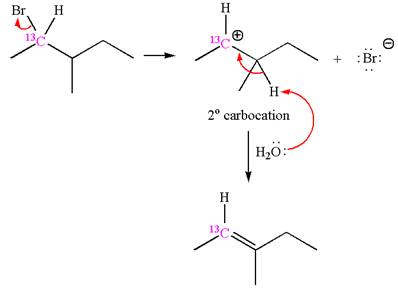
The reaction with carbocation rearrangement gave two products, and the reaction without carbocation rearrangement gave only one product, as shown below, indicating that the
Reaction with rearrangement:
Reaction without rearrangement:
Explanation of Solution
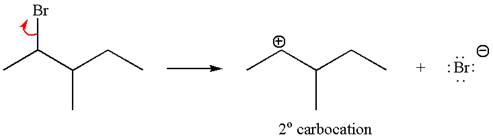
The given reaction equation is
If the carbon bonded to the leaving group in the given substrate is labelled as
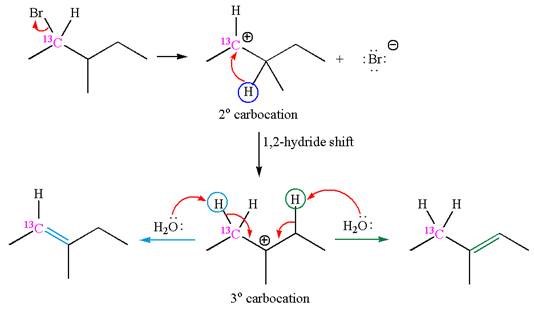
In one product, one of the double bonded carbon is
If the reaction proceeds without rearrangement, then only one product is formed where one of the double bonded carbon is
As the reaction with rearrangement of carbocation formed two products, and reaction without rearrangement formed only one product, it indicates that the E1 products depend on whether the rearrangement occurred.
It is explained that the E1 products depend on whether the reaction includes carbocation rearrangement occurring with
(d)
Interpretation:
How the deuterium isotope labeling is useful to determine whether the rearrangement is occurred in given
Concept introduction:
The
Answer to Problem 8.65P
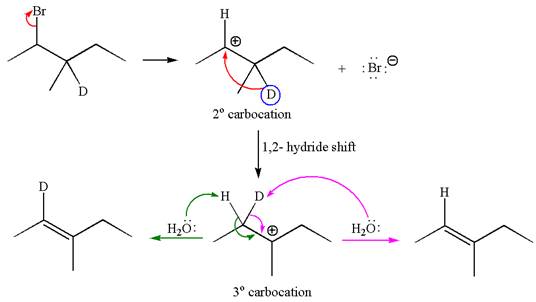
The reaction with carbocation rearrangement by migration of deuterium gave two products, and the reaction without carbocation rearrangement gave only one product as shown below, indicating that the deuterium isotope labeling is useful to determine whether the rearrangement occurred in the given
Reaction with rearrangement:
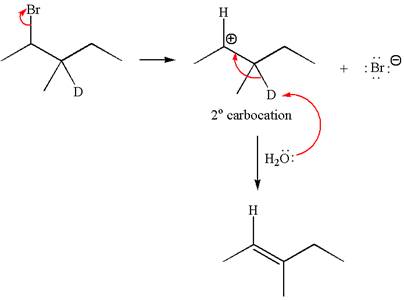
Reaction without rearrangement:
Explanation of Solution
The given reaction equation is
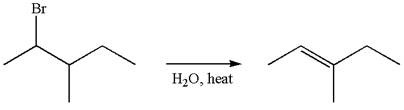
If the migrating hydrogen in the given substrate is replaced with deuterium, then two products are formed when the reaction occurred through carbocation rearrangement. The detailed mechanism is as follows:
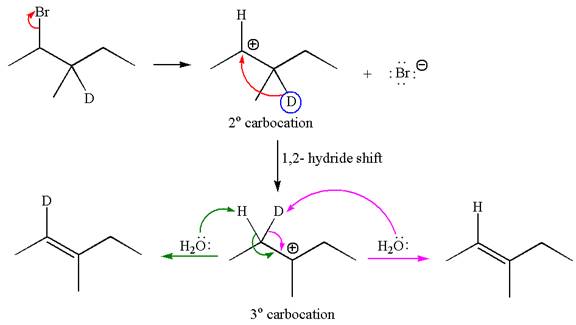
One product is formed by elimination of hydrogen atom and another by elimination of deuterium atom.
If the reaction proceeds without rearrangement, only one product is formed by elimination of deuterium atom. The detailed mechanism is as follows:
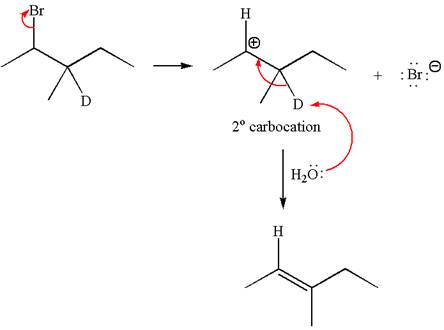
As the reaction with rearrangement of carbocation formed two products and reaction without rearrangement formed only one product, it indicates the E1 products depend on whether the rearrangement occurs.
It is explained on the basis of formation of different products that deuterium isotope labeling is useful to determine whether the rearrangement occurred in the given
Want to see more full solutions like this?
Chapter 8 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- In a reaction between two reactants A and B, the half-life is the same for both.(1) Only if the stoichiometry A:B is 1:1.(2) If the initial quantities of A and B are in their stoichiometric ratios.arrow_forwardThere are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Calculate the measurement of uncertainty and provide the data in a spreadsheet table. Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32 152.87 151.24 153.26 152.02 152.90 152.87 151.49 152.46 152.58arrow_forward1. Predict the organic product(s) of the following reactions. Assume excess of reagents unless otherwise noted. a) &l BH3 •THF b) 1) NaOH 2) H3O+ solve d) ala 1) EtMgBr 2) H3O+ e) H2N سكر CuLi NH2 1) SOCI2 2) EtMgBr 3) H3O+ NC H3O+ Δarrow_forward
- There are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Summarise and report these results including an indication of measurement uncertainty. In both calculation samples calculate if an outlier is present, max value, number of samples, mean, standard deviation, g (suspect), g (critical) and t (critical). Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32…arrow_forwardIndicate the rate expressions for reactions that have order 0, 1, and 2.arrow_forwardPROBLEMS Q1) Label the following salts as either acidic, basic, or neutral a) Fe(NOx) c) AlBr b) NH.CH COO d) HCOON (1/2 mark each) e) Fes f) NaBr Q2) What is the pH of a 0.0750 M solution of sulphuric acid?arrow_forward
- 8. Draw all the resonance forms for each of the fling molecules or ions, and indicate the major contributor in each case, or if they are equivalent (45) (2) -PH2 سمة مدarrow_forwardA J то گای ه +0 Also calculate the amount of starting materials chlorobenzaldehyde and p-chloroacetophenone required to prepare 400 mg of the given chalcone product 1, 3-bis(4-chlorophenyl)prop-2-en-1-one molar mass ok 1,3-bis(4-Chlorophenyl) prop-2-en-1-one = 277.1591m01 number of moles= 0.400/277.15 = 0.00144 moles 2 x 0.00 144=0.00288 moves arams of acetophenone = 0.00144 X 120.16 = 0.1739 0.1739x2=0.3469 grams of benzaldehyde = 0.00144X106.12=0.1539 0.1539x2 = 0.3069 Starting materials: 0.3469 Ox acetophenone, 0.3069 of benzaldehyde 3arrow_forward1. Answer the questions about the following reaction: (a) Draw in the arrows that can be used make this reaction occur and draw in the product of substitution in this reaction. Be sure to include any relevant stereochemistry in the product structure. + SK F Br + (b) In which solvent would this reaction proceed the fastest (Circle one) Methanol Acetone (c) Imagine that you are working for a chemical company and it was your job to perform a similar reaction to the one above, with the exception of the S atom in this reaction being replaced by an O atom. During the reaction, you observe the formation of three separate molecules instead of the single molecule obtained above. What is the likeliest other products that are formed? Draw them in the box provided.arrow_forward
- 3. For the reactions below, draw the arrows corresponding to the transformations and draw in the boxes the reactants or products as indicated. Note: Part A should have arrows drawn going from the reactants to the middle structure and the arrows on the middle structure that would yield the final structure. For part B, you will need to draw in the reactant before being able to draw the arrows corresponding to product formation. A. B. Rearrangement ΘΗarrow_forward2. Draw the arrows required to make the following reactions occur. Please ensure your arrows point from exactly where you want to exactly where you want. If it is unclear from where arrows start or where they end, only partial credit will be given. Note: You may need to draw in lone pairs before drawing the arrows. A. B. H-Br 人 C Θ CI H Cl Θ + Br Oarrow_forward4. For the reactions below, draw the expected product. Be sure to indicate relevant stereochemistry or formal charges in the product structure. a) CI, H e b) H lux ligh Br 'Harrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
