
Concept explainers
(a)
Interpretation:
The substitutive name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order.
Answer to Problem 8.5P
The substitutive name for the given compound is 1-butanol.
Explanation of Solution
The given compound is CH3CH2CH2CH2OH. It consists of a chain of four carbon atoms with hydroxyl group attached at the first carbon atom. The alcohols contain hydroxyl (OH)
The substitutive name for the given compound is 1-butanol.
(b)
Interpretation:
The substitutive name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order.
Answer to Problem 8.5P
The substitutive name for the given compound is 3-bromo-1-butanol.
Explanation of Solution
The given compound is CH3CHBrCH2CH2OH. It consists of a chain of four carbon atoms with hydroxyl group attached at the first carbon atom. The alcohols contain hydroxyl (OH) functional group in their parent chain and are named by adding suffix –ol. The bromo group is attached at the third carbon atom. Therefore, the name of this compound is 3-bromo-1-butanol.
The substitutive name for the given compound is 3-bromo-1-butanol.
(c)
Interpretation:
The substitutive name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order.
Answer to Problem 8.5P
The substitutive name for the given compound is (E)-1-chloro-3-methyl-3-pentene-2-ol.
Explanation of Solution
The given compound is shown below.
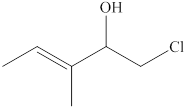
Figure 1
The given compound has a longest chain of five carbon atoms with a double bond on third carbon atom. It is an
The use of prefix E and Z depends upon the location of the higher priority groups. When the priority groups located on the opposite side then it is an E isomer, whereas when two higher priority groups located on the same side then it is an Z isomer. The priority order depends upon the
The substitutive name for the given compound is (E)-1-chloro-3-methyl-3-pentene-2-ol.
(d)
Interpretation:
The substitutive name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order.
Answer to Problem 8.5P
The substitutive name for the given compound is (1S,5R)-2-chloro-5-methylcyclopent-2-enethiol.
Explanation of Solution
The given compound is shown below.
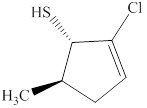
Figure 2
The given compound has a cyclic ring of five carbon atoms with a double bond on second carbon atom. It is an alkene, the suffix used for an alkene is –ene. Therefore, the parent name is cyclopent-2-ene. One chloro group is attached at the second carbon atom, one methyl group is attached at the fifth carbon atom and one thiol group is attached at the first carbon atom.
The naming of chiral center and geometric isomers are based on Cahn-Ingold-Prelog priority rules. If the priority assigned to each group attached to the chirality center in a molecule is in a clockwise direction, then it is the R-stereoisomer, and if this is counter-clockwise, then it is the S-stereoisomer. R and S-stereoisomer are mirror images of each other. Therefore, the name of this compound is (1S,5R)-2-chloro-5-methylcyclopent-2-enethiol.
The substitutive name for the given compound is (1S,5R)-2-chloro-5-methylcyclopent-2-enethiol.
(e)
Interpretation:
The substitutive name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order.
Answer to Problem 8.5P
The substitutive name for the given compound is (2R,3R,4S)-3-butylhex-5-ene-2, 4-diol.
Explanation of Solution
The given compound is shown below.
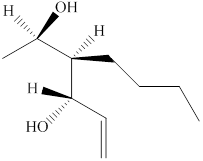
Figure 3
The given compound has a chain of six carbon atoms with a double bond on fifth carbon atom. It is an alkene, the suffix used for an alkene is –ene. Therefore, the parent name is hex-5-ene. One butyl group is attached at the third carbon atom and two hydroxyl groups are attached at the second and fourth carbon atoms.
The naming of chiral center and geometric isomers are based on Cahn-Ingold-Prelog priority rules. If the priority assigned to each group attached to the chirality center in a molecule is in a clockwise direction, then it is the R-stereoisomer, and if this is counter-clockwise, then it is the S-stereoisomer. R and S-stereoisomer are mirror images of each other. Therefore, the name of this compound is (2R,3R,4S)-3-butylhex-5-ene-2, 4-diol.
The substitutive name for the given compound is (2R,3R,4S)-3-butylhex-5-ene-2, 4-diol.
(f)
Interpretation:
The substitutive name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order.
Answer to Problem 8.5P
The substitutive name for the given compound is cyclohexa-2, 5-diene-1-ol.
Explanation of Solution
The given compound is shown below.
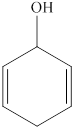
Figure 4
It consists of a cyclic ring of six carbon atoms. Therefore, the parent name is cyclohexa. There are two double bonds present at the second and fifth carbon atom of the ring. Also, one hydroxyl group is attached at the first carbon atom. Therefore, the name of this compound is cyclohexa-2, 5-diene-1-ol.
The substitutive name for the given compound is cyclohexa-2, 5-diene-1-ol.
(g)
Interpretation:
The substitutive name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order.
Answer to Problem 8.5P
The substitutive name for the given compound is 1-mercaptopentan-2-ol.
Explanation of Solution
The given compound is shown below.
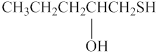
Figure 5
It consists of a chain of five carbon atoms with hydroxyl group attached at the second carbon atom. The alcohols contain hydroxyl (OH) functional group in their parent chain and are named by adding suffix –ol. The –SH group is attached at the first carbon atom. Therefore, the name of this compound is 1-mercaptopentan-2-ol.
The substitutive name for the given compound is 1-mercaptopentan-2-ol.
(h)
Interpretation:
The substitutive name for the given compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order.
Answer to Problem 8.5P
The substitutive name for the given compound is 2-methylpropan-2-thiol.
Explanation of Solution
The given compound is shown below.
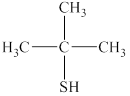
Figure 6
It consists of a chain of three carbon atoms with thiol group attached at the second carbon atom. One methyl group is attached at the second carbon atom. Therefore, the name of this compound is 2-methylpropan-2-thiol.
The substitutive name for the given compound is 2-methylpropan-2-thiol.
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- Please help me solve these two problems. Thank you in advance.arrow_forwardNaming and drawing unsubstituted esters Write the systematic name of each organic molecule: Explanation structure Check name Х 2/5arrow_forwardPredict the product of this organic reaction: =0 CH3-O-CH2-C-OH + CH3-OH H P+H₂O A Specifically, in the drawing area below draw the condensed structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. ☐arrow_forward
- Naming and drawing USUsted ester Draw the condensed structure of ethyl hexanoate. Click anywhere to draw the first atom of your structure. × A : ☐arrow_forwardExtra for Experts: Your Future in Chemistry. As you now know, there are countless jobs that involve chemistry! Research a chemistry profession that interests you. In your answer, discuss which aspects of the job most appeal to you.arrow_forwardMISSED THIS? Read Section 19.9 (Pages 878-881); Watch IWE 19.10 Consider the following reaction: CH3OH(g) CO(g) + 2H2(g) (Note that AG,CH3OH(g) = -162.3 kJ/mol and AG,co(g)=-137.2 kJ/mol.) Part A Calculate AG for this reaction at 25 °C under the following conditions: PCH₂OH Pco PH2 0.815 atm = 0.140 atm 0.170 atm Express your answer in kilojoules to three significant figures. Ο ΑΣΦ AG = -150 Submit Previous Answers Request Answer □? kJ × Incorrect; Try Again; 2 attempts remaining Calculate the free energy change under nonstandard conditions (AGrxn) by using the following relationship: AGrxn = AGrxn + RTInQ, AGxn+RTInQ, where AGxn is the standard free energy change, R is the ideal gas constant, T is the temperature in kelvins, a is the reaction quotient. Provide Feedback Next >arrow_forward
- Identify and provide a brief explanation of Gas Chromatography (GC) within the context of chemical analysis of food. Incorporate the specific application name, provide a concise overview of sample preparation methods, outline instrumental parameters and conditions ultilized, and summarise the outcomes and findings achieved through this analytical approach.arrow_forwardIdentify and provide a concise explanation of the concept of signal-to-noise ratio (SNR) in the context of chemical analysis. Provide specific examples.arrow_forwardIdentify and provide a concise explanation of a specific analytical instrument capable of detecting and quantifying trace compounds in food samples. Emphasise the instrumental capabilities relevant to trace compound analysis in the nominated food. Include the specific application name (eg: identification and quantification of mercury in salmon), outline a brief description of sample preparation procedures, and provide a summary of the obtained results from the analytical process.arrow_forward
- Identify and provide an explanation of what 'Seperation Science' is. Also describe its importance with the respect to the chemical analysis of food. Provide specific examples.arrow_forward5. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn. H3C CH3arrow_forwardState the name and condensed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forward

 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning



