
GENERAL, ORGANIC, BIOCHEM (LL W/ ACCESS)
10th Edition
ISBN: 9781260885958
Author: Denniston
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 8.43QP
Interpretation Introduction
Interpretation:
The stronger base between nitrate ion and cyanide ion has to be identified.
Concept Introduction:
Acids and bases classified as strong when the reaction with water undergoes 100% completion and as weak when the reaction with water is much less than 100% complete. The relative strength of Acid-base are given by
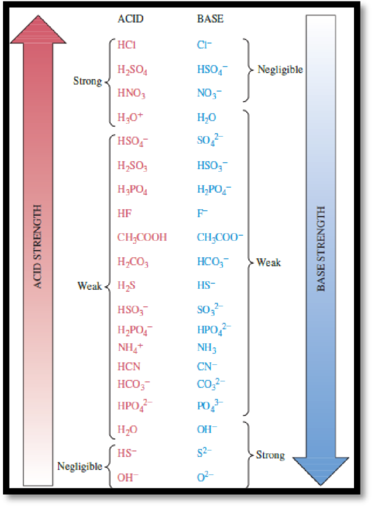
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please help me answer a. Please and thank you I advance.
Draw both of the chair flips for both the cis and trans isomers for the following
compounds:
1,4-diethylcyclohexane
1-methyl-3-secbutylcyclohexane
Ppplllleeeaaasssseeee hellppp wiithhh thisss physical chemistryyyyy
I talked like this because AI is very annoying
Chapter 8 Solutions
GENERAL, ORGANIC, BIOCHEM (LL W/ ACCESS)
Ch. 8.1 - Classify CH3COO− as a Brønsted-Lowry acid or base,...Ch. 8.1 - Prob. 8.1QCh. 8.1 - Prob. 8.2QCh. 8.1 - Write an equation for the reversible reactions of...Ch. 8.1 - Prob. 8.4QCh. 8.1 - Prob. 8.5QCh. 8.1 - Prob. 8.6QCh. 8.1 - Prob. 8.2PPCh. 8.1 - Analysis of a patient’s blood sample indicated...Ch. 8.1 - Prob. 8.7Q
Ch. 8.1 - The hydroxide ion concentration in a sample of...Ch. 8.2 - Calculate the pH of a 1.0 × 10−4 M solution of...Ch. 8.2 - Calculate the [H3O+] of a solution of HNO3 that...Ch. 8.2 - Calculate the pH corresponding to a 1.0 × 10−2 M...Ch. 8.2 - Calculate the [H3O+] and [OH−] of a potassium...Ch. 8.2 - Calculate the [H3O+] corresponding to pH =...Ch. 8.2 - Prob. 8.9PPCh. 8.2 - Calculate the [OH–] of a 1.0 × 10–3 M solution of...Ch. 8.2 - Prob. 8.10QCh. 8.3 - Calculate the molar concentration of a sodium...Ch. 8.4 - A buffer solution is prepared in such a way that...Ch. 8.4 - Prob. 8.12PPCh. 8.4 - Prob. 8.11QCh. 8.4 - Prob. 8.12QCh. 8.4 - Prob. 8.13QCh. 8.4 - Prob. 8.14QCh. 8.4 - Prob. 8.15QCh. 8.4 - Prob. 8.16QCh. 8.4 - Prob. 8.17QCh. 8.4 - Explain how the pH of blood would change under...Ch. 8.4 - Write the Henderson-Hasselbalch expression for the...Ch. 8.4 - Prob. 8.20QCh. 8.5 - Prob. 8.21QCh. 8.5 - Prob. 8.22QCh. 8.5 - Prob. 8.23QCh. 8.5 - Prob. 8.24QCh. 8.5 - Chrome plating involves the reduction of Cr3+(aq)...Ch. 8.5 - Prob. 8.26QCh. 8 - Prob. 8.27QPCh. 8 - Define a base according to the Arrhenius...Ch. 8 - What are the essential differences between the...Ch. 8 - Why is ammonia described as a Brønsted-Lowry base...Ch. 8 - Classify each of the following as either a...Ch. 8 - Classify each of the following as either a...Ch. 8 - Classify each of the following as either a...Ch. 8 - Classify each of the following as either a...Ch. 8 - Write an equation for the reaction of each of the...Ch. 8 - Write an equation for the reaction of each of the...Ch. 8 - Write the formula of the conjugate acid of CN−.
Ch. 8 - Write the formula of the conjugate acid of Br−.
Ch. 8 - Write the formula of the conjugate base of HI.
Ch. 8 - Write the formula of the conjugate base of HCOOH.
Ch. 8 - Write the formula of the conjugate acid of NO3−.
Ch. 8 - Write the formula of the conjugate acid of F−.
Ch. 8 - Which is the stronger base, NO3− or CN−?
Ch. 8 - Prob. 8.44QPCh. 8 - Prob. 8.45QPCh. 8 - Which is the stronger base, F− or CH3COO−?
Ch. 8 - Identify the conjugate acid-base pairs in each of...Ch. 8 - Identify the conjugate acid-base pairs in each of...Ch. 8 - Distinguish between the terms acid-base strength...Ch. 8 - Label each of the following as a strong or weak...Ch. 8 - Label each of the following as a strong or weak...Ch. 8 - Calculate the [H3O+] of an aqueous solution that...Ch. 8 - Calculate the [H3O+] of an aqueous solution that...Ch. 8 - Calculate the [OH−] of an aqueous solution that...Ch. 8 - Prob. 8.56QPCh. 8 - Prob. 8.57QPCh. 8 - What is the concentration of hydronium ions in an...Ch. 8 - Prob. 8.59QPCh. 8 - Consider two beakers, one containing 0.10 M NaOH...Ch. 8 - Calculate the pH of a solution that is:
1.0 × 10−2...Ch. 8 - Calculate the pH of a solution that is:
1.0 × 10−1...Ch. 8 - Calculate [H3O+] for a solution of nitric acid for...Ch. 8 - Calculate [H3O+] for a solution of hydrochloric...Ch. 8 - Prob. 8.65QPCh. 8 - Prob. 8.66QPCh. 8 - Calculate both [H3O+] and [OH−] for a solution for...Ch. 8 - Calculate both [H3O+] and [OH−] for a solution for...Ch. 8 - What is a neutralization reaction?
Ch. 8 - Describe the purpose of a titration.
Ch. 8 - Prob. 8.71QPCh. 8 - The pH of urine may vary between 4.5 and 8.2....Ch. 8 - Criticize the following statement: A lakewater...Ch. 8 - Can a dilute solution of a strong acid ever have a...Ch. 8 - What is the H3O+ concentration of a solution with...Ch. 8 - What is the H3O+ concentration of a solution with...Ch. 8 - Prob. 8.77QPCh. 8 - Prob. 8.78QPCh. 8 - Calculate the pH of a solution that has [H3O+] =...Ch. 8 - Calculate the pH of a solution that has [H3O+] =...Ch. 8 - Calculate the pH of a solution that has [OH−] =...Ch. 8 - Calculate the pH of a solution that has [OH−] =...Ch. 8 - Prob. 8.83QPCh. 8 - Prob. 8.84QPCh. 8 - Prob. 8.85QPCh. 8 - Prob. 8.86QPCh. 8 - Write an equation to represent the neutralization...Ch. 8 - Write an equation to represent the neutralization...Ch. 8 - Prob. 8.89QPCh. 8 - Prob. 8.90QPCh. 8 - Prob. 8.91QPCh. 8 - Prob. 8.92QPCh. 8 - Titration of 15.00 mL of HCl solution requires...Ch. 8 - Titration of 17.85 mL of HNO3 solution requires...Ch. 8 - Prob. 8.95QPCh. 8 - Prob. 8.96QPCh. 8 - Prob. 8.97QPCh. 8 - Prob. 8.98QPCh. 8 - Which of the following are capable of forming a...Ch. 8 - Which of the following are capable of forming a...Ch. 8 - Prob. 8.101QPCh. 8 - Prob. 8.102QPCh. 8 - Prob. 8.103QPCh. 8 - Prob. 8.104QPCh. 8 - For the equilibrium situation involving acetic...Ch. 8 - Prob. 8.106QPCh. 8 - Prob. 8.107QPCh. 8 - Prob. 8.108QPCh. 8 - Prob. 8.109QPCh. 8 - For the buffer system described in Question 8.105,...Ch. 8 - Prob. 8.111QPCh. 8 - Prob. 8.112QPCh. 8 - Prob. 8.113QPCh. 8 - Prob. 8.114QPCh. 8 - Prob. 8.115QPCh. 8 - Prob. 8.116QPCh. 8 - Prob. 8.117QPCh. 8 - Prob. 8.118QPCh. 8 - In the following reaction, identify the oxidized...Ch. 8 - Prob. 8.120QPCh. 8 - Prob. 8.121QPCh. 8 - Prob. 8.122QPCh. 8 - Prob. 8.123QPCh. 8 - Prob. 8.124QPCh. 8 - Prob. 8.125QPCh. 8 - Prob. 8.126QPCh. 8 - Prob. 1MCPCh. 8 - Prob. 2MCPCh. 8 - Prob. 3MCPCh. 8 - Prob. 4MCPCh. 8 - Prob. 5MCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For this question, if the product is racemic, input both enantiomers in the same Marvin editor. A) Input the number that corresponds to the reagent which when added to (E)-but-2-ene will result in a racemic product. Input 1 for Cl, in the cold and dark Input 2 for Oy followed by H₂O, Zn Input 3 for D₂ with metal catalyst Input 4 for H₂ with metal catalyst B) Draw the skeletal structure of the major organic product made from the reagent in part A Marvin JS Help Edit drawing C) Draw the skeletal structure of the major organic product formed when (2)-but-2-ene is treated with peroxyacetic acid. Marvin 35 Helparrow_forwardMichael Reactions 19.52 Draw the products from the following Michael addition reactions. 1. H&C CH (a) i 2. H₂O* (b) OEt (c) EtO H₂NEt (d) ΕΙΟ + 1. NaOEt 2. H₂O' H H 1. NaOEt 2. H₂O*arrow_forwardRank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic. НОН НЬ OHd Онсarrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? ? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C :0 T Add/Remove step Garrow_forwardThe following equations represent the formation of compound MX. What is the AH for the electron affinity of X (g)? X₂ (g) → 2X (g) M (s) → M (g) M (g) M (g) + e- AH = 60 kJ/mol AH = 22 kJ/mol X (g) + e-X (g) M* (g) +X (g) → MX (s) AH = 118 kJ/mol AH = ? AH = -190 kJ/mol AH = -100 kJ/mol a) -80 kJ b) -30 kJ c) -20 kJ d) 20 kJ e) 156 kJarrow_forwardA covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forward
- Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forwardAll of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forward
- A student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forwardCalculate the density of 21.12 g of an object that displaces 0.0250 L of water.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY