
(a)
Interpretation:
The number of electrons in an atom with the
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
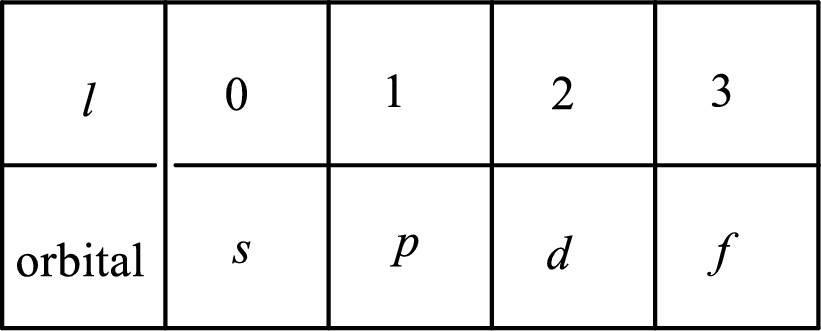
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(b)
Interpretation:
The number of electrons in an atom with
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
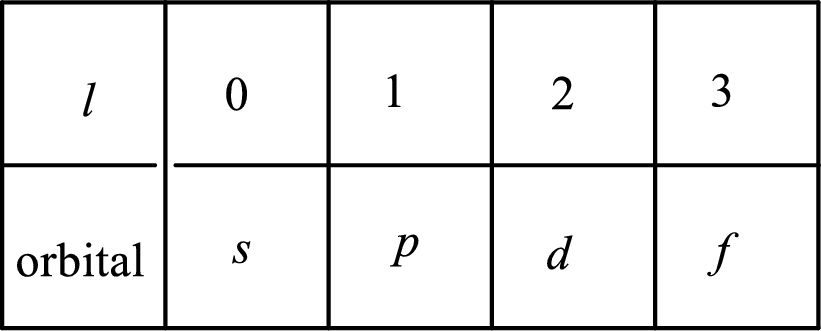
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(c)
Interpretation:
The number of electrons in an atom with
Concept introduction:
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
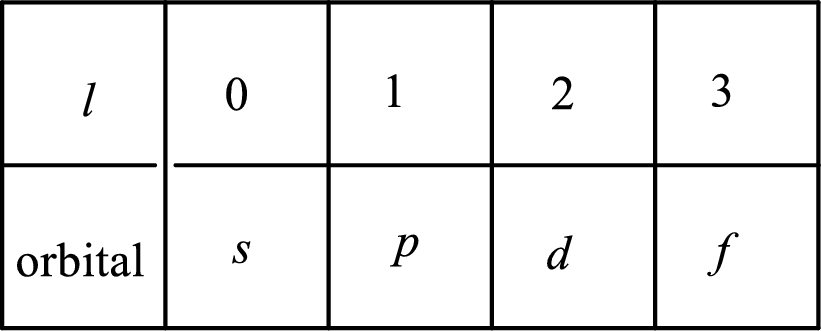
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
CHEMISTRY MOLECULAR NATURE OF MATTER
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





