
Chemistry
7th Edition
ISBN: 9780321940872
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 8.11P
Interpretation Introduction
To determine:
Whether
Interpretation Introduction
To determine:
Whether 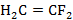 have a dipole moment, and the direction dipole moment to be determined.
have a dipole moment, and the direction dipole moment to be determined.
Interpretation Introduction
To determine:
Whether
Interpretation Introduction
To determine:
Whether
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
20. The Brusselator. This hypothetical system was first proposed by a group work-
ing in Brussels [see Prigogine and Lefever (1968)] in connection with spatially
nonuniform chemical patterns. Because certain steps involve trimolecular reac
tions, it is not a model of any real chemical system but rather a prototype that
has been studied extensively. The reaction steps are
A-X.
B+X-Y+D.
2X+ Y-3X,
X-E.
305
It is assumed that concentrations of A, B, D, and E are kept artificially con
stant so that only X and Y vary with time.
(a) Show that if all rate constants are chosen appropriately, the equations de
scribing a Brusselator are:
dt
A-(B+ 1)x + x²y,
dy
=Bx-x²y.
di
Problem 3. Provide a mechanism for the following transformation:
H₂SO A
Me.
Me
Me
Me
Me
You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other major side products:
xi
1. ☑
2. H₂O
хе
i
Draw the missing reagent X you think will make this synthesis work in the drawing area below.
If there is no reagent that will make your desired product in good yield or without complications, just check the box under the drawing area and leave it blank.
Click and drag to start drawing a
structure.
There is no reagent that will make this synthesis work without complications.
: ☐
S
☐
Chapter 8 Solutions
Chemistry
Ch. 8 - Prob. 8.1PCh. 8 - Prob. 8.2ACh. 8 - PRACTICE 8.3 Acetic acid, CH3CO2H , is the main...Ch. 8 - APPLY 8.4 Benzene, C6H6 , is a cyclic molecule in...Ch. 8 - PRACTICE 8.5 Identify the orbitals that overlap to...Ch. 8 - APPLY 8.6 Describe the bonding in propane, C3H8 ,...Ch. 8 - PRACTICE 8.7 Describe the hybridization of the...Ch. 8 - APPLY 8.8 Describe the hybridization of each...Ch. 8 - PRACTICE 8.9 Describe the hybridization of the...Ch. 8 - APPLY 8.10 Describe the hybridization of the...
Ch. 8 - Prob. 8.11PCh. 8 - Conceptual APPLY 8.12 Match the following...Ch. 8 - Prob. 8.13PCh. 8 - Prob. 8.14ACh. 8 - Prob. 8.15PCh. 8 - Prob. 8.16ACh. 8 - Prob. 8.17PCh. 8 - Prob. 8.18ACh. 8 - Prob. 8.19PCh. 8 - Prob. 8.20ACh. 8 - Prob. 8.21PCh. 8 - Prob. 8.22ACh. 8 - PRACTICE 8.23 Draw two resonance structures for...Ch. 8 - APPLY 8.24 Draw two resonance structures for the...Ch. 8 - Prob. 8.25PCh. 8 - Prob. 8.26PCh. 8 - PROBLEM 8.27 Identify which of the following...Ch. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - Prob. 8.30PCh. 8 - Prob. 8.31CPCh. 8 - Prob. 8.32CPCh. 8 - Prob. 8.33CPCh. 8 - Prob. 8.34CPCh. 8 - Prob. 8.35CPCh. 8 - Prob. 8.36CPCh. 8 - Prob. 8.37CPCh. 8 - Prob. 8.38CPCh. 8 - Prob. 8.39CPCh. 8 - Prob. 8.40CPCh. 8 - Two dichioroethylene molecules with the same...Ch. 8 - Prob. 8.42SPCh. 8 - Prob. 8.43SPCh. 8 - Prob. 8.44SPCh. 8 - How many charge clouds are there around the...Ch. 8 - Prob. 8.46SPCh. 8 - Prob. 8.47SPCh. 8 - What shape do you expect for each of the following...Ch. 8 - Prob. 8.49SPCh. 8 - Prob. 8.50SPCh. 8 - Prob. 8.51SPCh. 8 - Prob. 8.52SPCh. 8 - Prob. 8.53SPCh. 8 - Acrylonitrile is used as the starting material for...Ch. 8 - Predict values for all bond angles in dimethyl...Ch. 8 - Oceanographers study the mixing of water masses by...Ch. 8 - Prob. 8.57SPCh. 8 - Prob. 8.58SPCh. 8 - Prob. 8.59SPCh. 8 - Prob. 8.60SPCh. 8 - Prob. 8.61SPCh. 8 - Prob. 8.62SPCh. 8 - Prob. 8.63SPCh. 8 - Prob. 8.64SPCh. 8 - Prob. 8.65SPCh. 8 - Oxaloacetic acid is an intermediate involved in...Ch. 8 - The atoms in the amino acid glycine are connected...Ch. 8 - Prob. 8.68SPCh. 8 - Prob. 8.69SPCh. 8 - Prob. 8.70SPCh. 8 - 8.71 What is the difference between London...Ch. 8 - 8.72 What are the most important kinds of...Ch. 8 - Of the substances Xe,CH3Cl,HF, which has: (a) The...Ch. 8 - 8.74 Methanol boils nearlyhigher than methane, but...Ch. 8 - Prob. 8.75SPCh. 8 - Prob. 8.76SPCh. 8 - Prob. 8.77SPCh. 8 - Prob. 8.78SPCh. 8 - Prob. 8.79SPCh. 8 - Prob. 8.80SPCh. 8 - 8.81 Draw three-dimensional structures of PCl3 and...Ch. 8 - Prob. 8.82SPCh. 8 - Prob. 8.83SPCh. 8 - Prob. 8.84SPCh. 8 - Prob. 8.85SPCh. 8 - 8.86 A liquid sample contains methylamine (CH3NH2)...Ch. 8 - Prob. 8.87SPCh. 8 - Prob. 8.88SPCh. 8 - Prob. 8.89SPCh. 8 - Prob. 8.90SPCh. 8 - Prob. 8.91SPCh. 8 - Prob. 8.92SPCh. 8 - Prob. 8.93SPCh. 8 - Prob. 8.94SPCh. 8 - Prob. 8.95SPCh. 8 - Prob. 8.96SPCh. 8 - Prob. 8.98CPCh. 8 - Prob. 8.99CPCh. 8 - Prob. 8.100CPCh. 8 - Prob. 8.101CPCh. 8 - Prob. 8.102CPCh. 8 - Prob. 8.103CPCh. 8 - Prob. 8.104CPCh. 8 - Prob. 8.105CPCh. 8 - Prob. 8.106CPCh. 8 - Prob. 8.107CPCh. 8 - Prob. 8.108CPCh. 8 - Prob. 8.109CPCh. 8 - The odor of cinnamon oil is due to cinnamaldehyde,...Ch. 8 - Prob. 8.111CPCh. 8 - Prob. 8.112CPCh. 8 - Prob. 8.113CPCh. 8 - Prob. 8.114CPCh. 8 - Prob. 8.115CPCh. 8 - Prob. 8.116CPCh. 8 - Prob. 8.117CPCh. 8 - Prob. 8.118CPCh. 8 - Prob. 8.119MPCh. 8 - Prob. 8.120MPCh. 8 - Prob. 8.121MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of this organic reaction: H OH 1. LiAlH4 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. G C टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 CI MgCl ? Will the first product that forms in this reaction create a new CC bond? Yes No MgBr ? Will the first product that forms in this reaction create a new CC bond? Yes No G टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forward
- Predict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forwardPredict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forward
- As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forwardgive example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward
- 2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward(Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY