
(a)
Interpretation: The plot for the given seven ionization energies (IE) for the given atom, the shell that has electrons corresponds to the respective IE and the reason for presence of the break in graph should be determined.
Concept Introduction:
Cation: Removal of electron from the atom results to form positively charged ion called cation.
Anion: Addition of electron to atom results to form negatively charged ion called anion.
The net charge present in the element denotes the presence or absence of electrons in the element.
First ionization energy:
The ionization energy is the minimum energy required to remove the electron from an isolated atom which is in the gaseous state results to give gaseous ion with one positive charge.
Second ionization:
Repeating the same process that is removal of another electron that is second electron from the resulting ion of first ionization is called second ionization.
Third ionization energy:
Removal of electron from ion that results from the second ionization is called third ionization which results to give ion with three positive charges which shows, three electrons gets removed from the atom and the energy associated with it is called third ionization energy.
To plot: The graph considering the given ionization energies for the given element.
(a)
Answer to Problem 8.109QP
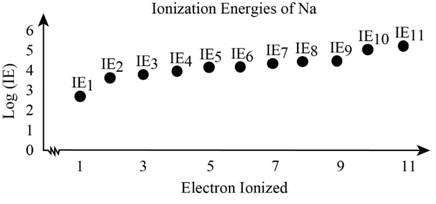
Analyze the given data and plot the data for the given element.
Explanation of Solution
The graph that shows the ionization energies for the given elements is drawn considering the given data.
The ionization energy increases since it involves removal of electrons such the successive removal of electrons requires more energy compared to the previous as core electrons tends to tightly bound with the nucleus.
(b)
Interpretation: The plot for the given seven ionization energies (IE) for the given atom, the shell that has electrons corresponds to the respective IE and the reason for presence of the break in graph should be determined.
Concept Introduction:
Cation: Removal of electron from the atom results to form positively charged ion called cation.
Anion: Addition of electron to atom results to form negatively charged ion called anion.
The net charge present in the element denotes the presence or absence of electrons in the element.
First ionization energy:
The ionization energy is the minimum energy required to remove the electron from an isolated atom which is in the gaseous state results to give gaseous ion with one positive charge.
Second ionization:
Repeating the same process that is removal of another electron that is second electron from the resulting ion of first ionization is called second ionization.
Third ionization energy:
Removal of electron from ion that results from the second ionization is called third ionization which results to give ion with three positive charges which shows, three electrons gets removed from the atom and the energy associated with it is called third ionization energy.
To determine: The orbital that contains the electron with respect to the energy.
(b)
Explanation of Solution
Examining the graph shows that first the valence electron in the given element gets removed which present in the
The orbital that contains electrons with respect to the given energy is determined as above.
(c)
Interpretation: The plot for the given seven ionization energies (IE) for the given atom, the shell that has electrons corresponds to the respective IE and the reason for presence of the break in graph should be determined.
Concept Introduction:
Cation: Removal of electron from the atom results to form positively charged ion called cation.
Anion: Addition of electron to atom results to form negatively charged ion called anion.
The net charge present in the element denotes the presence or absence of electrons in the element.
First ionization energy:
The ionization energy is the minimum energy required to remove the electron from an isolated atom which is in the gaseous state results to give gaseous ion with one positive charge.
Second ionization:
Repeating the same process that is removal of another electron that is second electron from the resulting ion of first ionization is called second ionization.
Third ionization energy:
Removal of electron from ion that results from the second ionization is called third ionization which results to give ion with three positive charges which shows, three electrons gets removed from the atom and the energy associated with it is called third ionization energy.
To determine: The reason for the breaks in the curve of the plot for given element.
(c)
Explanation of Solution
The break in the curve of the plot explains that removal of electrons from one energy level to other level that is break is appeared two times one with going from third to 2nd level and the other from 2nd to the first energy level.
Want to see more full solutions like this?
Chapter 8 Solutions
CHEMISTRY (LOOSELEAF) >CUSTOM<
- Draw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forward
- Differentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forward
- Answer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





