
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
9th Edition
ISBN: 9781305020788
Author: John C.Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 80GQ
Amides are an important class of organic molecules. They are usually drawn as sketched here, but another resonance structure is possible.
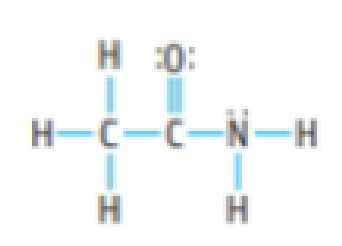
- (a) Draw a second resonance structure of the structure above. Do you think that this should be a significant contributor to the bonding? Explain your answer.
- (b) The H—N—H angle is close to 120° in this molecule. Does this fact influence your thoughts on the importance of this structure?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
what are the Iupac names for each structure
What are the IUPAC Names of all the compounds in the picture?
1) a) Give the dominant Intermolecular Force (IMF) in a sample of each of the following
compounds. Please show your work. (8) SF2, CH,OH, C₂H₂
b) Based on your answers given above, list the compounds in order of their Boiling Point
from low to high. (8)
Chapter 8 Solutions
CHEMISTRY+CHEM...HYBRID ED.(LL)>CUSTOM<
Ch. 8.2 - Draw Lewis electron dot structures for CH3Cl...Ch. 8.2 - Prob. 2CYUCh. 8.2 - Prob. 3CYUCh. 8.2 - Prob. 4CYUCh. 8.2 - Prob. 1RCCh. 8.2 - 2. Which one of the species in the list below is...Ch. 8.2 - Prob. 3RCCh. 8.2 - Prob. 4RCCh. 8.3 - Prob. 1CYUCh. 8.3 - 1. What is the formal charge of the P atom in the...
Ch. 8.4 - Draw resonance structures for the bicarbonate ion,...Ch. 8.4 - 1. For which of the following species, SO32−, NO+,...Ch. 8.4 - Prob. 2RCCh. 8.5 - Sketch the Lewis structures for CIF2+ and CIF2....Ch. 8.5 - Prob. 1QCh. 8.5 - Prob. 2QCh. 8.5 - Prob. 1RCCh. 8.5 - Prob. 2RCCh. 8.6 - What is the shape of the dichloromethane (CH2C12)...Ch. 8.6 - Give the electron-pair geometry and molecular...Ch. 8.6 - Draw the Lewis structure for lCl2, and then decide...Ch. 8.6 - Prob. 4CYUCh. 8.6 - Which of the following species has...Ch. 8.6 - Prob. 2RCCh. 8.6 - What is the approximate ClCCl bond angle in...Ch. 8.6 - 4. What is the molecular geometry of N2O (where...Ch. 8.7 - Draw the resonance structures for SCN. What are...Ch. 8.7 - For each of the following molecules, decide...Ch. 8.7 - Prob. 1RCCh. 8.7 - 2. Which of the following best describes the...Ch. 8.7 - Three resonance forms can be drawn for the...Ch. 8.8 - The electrostatic potential surface for SOCl2 is...Ch. 8.8 - Using the bond dissociation enthalpies in Table...Ch. 8.8 - Prob. 1RCCh. 8.8 - Prob. 2RCCh. 8.9 - 1. Which of the following species has the longest...Ch. 8.9 - 2. Which of the following species has the largest...Ch. 8.9 - 3. Use bond dissociation enthalpies to estimate...Ch. 8 - Give the periodic group number and number of...Ch. 8 - Give the periodic group number and number of...Ch. 8 - For elements in Groups 4A-7A of the periodic...Ch. 8 - Prob. 4PSCh. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Show all possible resonance structures for each of...Ch. 8 - Show all possible resonance structures for each of...Ch. 8 - Prob. 11PSCh. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Determine the formal charge on each atom in the...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Prob. 18PSCh. 8 - Prob. 19PSCh. 8 - The following molecules or ions all have three...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Draw a Lewis structure for each of the following...Ch. 8 - Give approximate values for the indicated bond...Ch. 8 - Give approximate values for the indicated bond...Ch. 8 - Phenylalanine is one of the natural amino acids...Ch. 8 - Acetylacetone has the structure shown here....Ch. 8 - For each pair of bonds, indicate the more polar...Ch. 8 - For each of the bonds listed below, tell which...Ch. 8 - Urea, (NH2)2CO, is used in plastics and...Ch. 8 - Considering both formal charges and bond...Ch. 8 - Considering both formal charge and bond...Ch. 8 - Three resonance structures are possible for...Ch. 8 - Three resonance structures are possible for the...Ch. 8 - Compare the electron dot structures of the...Ch. 8 - Compare the electron dot structures of the...Ch. 8 - The chemistry of the nitrite ion and HNO2: (a) Two...Ch. 8 - Draw the resonance structures for the formate ion,...Ch. 8 - Prob. 39PSCh. 8 - Consider the following molecules: (a) CH4 (b)...Ch. 8 - Which of the following molecules is(are) polar?...Ch. 8 - Prob. 42PSCh. 8 - Give the bond order for each bond in the following...Ch. 8 - Prob. 44PSCh. 8 - In each pair of bonds, predict which is shorter....Ch. 8 - In each pair of bonds, predict which is shorter....Ch. 8 - Prob. 47PSCh. 8 - Compare the carbon-oxygen bond lengths in the...Ch. 8 - Consider the carbon-oxygen bond in formaldehyde...Ch. 8 - Compare the nitrogen-nitrogen bond in hydrazine,...Ch. 8 - Ethanol can be made by the reaction of ethylene...Ch. 8 - Methanol can be made by partial oxidation of...Ch. 8 - Hydrogenation reactions, which involve the...Ch. 8 - Phosgene, Cl2CO, is a highly toxic gas that was...Ch. 8 - The compound oxygen difluoride is quite reactive,...Ch. 8 - Oxygen atoms can combine with ozone to form...Ch. 8 - Prob. 57GQCh. 8 - Prob. 58GQCh. 8 - Which of the following compounds or ions do not...Ch. 8 - Prob. 60GQCh. 8 - Draw resonance structures for the formate ion,...Ch. 8 - Prob. 62GQCh. 8 - Prob. 63GQCh. 8 - What is the principle of electroneutrality? Use...Ch. 8 - Prob. 65GQCh. 8 - Draw resonance structures for the SO2 molecule,...Ch. 8 - What are the orders of the NO bonds in NO2 and...Ch. 8 - Which has the greater ONO bond angle, NO2 or NO2+?...Ch. 8 - Compare the FClF angles in CIF2+ and ClF2. Using...Ch. 8 - Draw an electron dot structure for the cyanide...Ch. 8 - Draw the electron dot structure for the sulfite...Ch. 8 - Dinitrogen monoxide, N2O, can decompose to...Ch. 8 - The equation for the combustion of gaseous...Ch. 8 - The cyanate ion, OCN, has the least...Ch. 8 - Vanillin is the flavoring agent in vanilla extract...Ch. 8 - Explain why (a) XeF2 has a linear molecular...Ch. 8 - The formula for nitryl chloride is ClNO2 (in which...Ch. 8 - Hydroxyproline is a less-common amino acid. (a)...Ch. 8 - Amides are an important class of organic...Ch. 8 - Prob. 81GQCh. 8 - The molecule shown here. 2-furylmelhanethiol, is...Ch. 8 - Dihydroxyacetone is a component of quick-tanning...Ch. 8 - It is possible to draw three resonance structures...Ch. 8 - Acrolein is used to make plastics. Suppose this...Ch. 8 - Molecules in space: (a) In addition to molecules...Ch. 8 - 1,2-Dichloroethylene can be synthesized by adding...Ch. 8 - The molecule pictured below is epinephrine, a...Ch. 8 - You are doing an experiment in the laboratory and...Ch. 8 - Prob. 90ILCh. 8 - A paper published in the research Journal Science...Ch. 8 - Uracil is one of the bases in RNA, a close...Ch. 8 - Prob. 93SCQCh. 8 - Prob. 94SCQCh. 8 - Bromine-containing species play a role in...Ch. 8 - Acrylamide, H2C=CHCONH2, is a known neurotoxin and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 19.78 Write the products of the following sequences of reactions. Refer to your reaction road- maps to see how the combined reactions allow you to "navigate" between the different functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18 roadmaps along with your new Chapter 19 roadmap for these. (a) 1. BHS 2. H₂O₂ 3. H₂CrO4 4. SOCI₂ (b) 1. Cl₂/hv 2. KOLBU 3. H₂O, catalytic H₂SO4 4. H₂CrO4 Reaction Roadmap An alkene 5. EtOH 6.0.5 Equiv. NaOEt/EtOH 7. Mild H₂O An alkane 1.0 2. (CH3)₂S 3. H₂CrO (d) (c) 4. Excess EtOH, catalytic H₂SO OH 4. Mild H₂O* 5.0.5 Equiv. NaOEt/EtOH An alkene 6. Mild H₂O* A carboxylic acid 7. Mild H₂O* 1. SOC₁₂ 2. EtOH 3.0.5 Equiv. NaOEt/E:OH 5.1.0 Equiv. NaOEt 6. NH₂ (e) 1. 0.5 Equiv. NaOEt/EtOH 2. Mild H₂O* Br (f) i H An aldehyde 1. Catalytic NaOE/EtOH 2. H₂O*, heat 3. (CH,CH₂)₂Culi 4. Mild H₂O* 5.1.0 Equiv. LDA Br An ester 4. NaOH, H₂O 5. Mild H₂O* 6. Heat 7. MgBr 8. Mild H₂O* 7. Mild H₂O+arrow_forwardLi+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water? Group of answer choices LiBr LiI LiF LiClarrow_forwardQ4: Write organic product(s) of the following reactions and show the curved-arrow mechanism of the reactions. Br MeOH OSO2CH3 MeOHarrow_forward
- Provide the correct IUPAC name for the compound shown here. Reset cis- 5- trans- ☑ 4-6- 2- 1- 3- di iso tert- tri cyclo sec- oct but hept prop hex pent yl yne ene anearrow_forwardQ6: Predict the major product(s) for the following reactions. Note the mechanism (SN1, SN2, E1 or E2) the reaction proceeds through. If no reaction takes place, indicate why. Pay attention to stereochemistry. NaCN DMF Br σ Ilm... Br H Br H H NaCN CH3OH KOtBu tBuOH NaBr H₂O LDA Et2O (CH3)2CHOH KCN DMSO NaOH H₂O, A LDA LDA Systemarrow_forwardQ7: For the following reactions, indicate the reaction conditions that would provide the indicated product in a high yield. Note the major reaction pathway that would take place (SN1, SN2, E1, or E2) Note: There may be other products that are not shown. There maybe more than one plausible pathway. Br H3C OH H3C CI ... H3C SCH2CH3 CI i SCH2CH3 ཨ་ Br System Settarrow_forward
- Q2: Rank the compounds in each of the following groups in order of decreasing rate of solvolysis in aqueous acetone. OSO2CF3 OSO2CH3 OH a. b. CI Brarrow_forwardох 4-tert-butyl oxy cyclohex-1-ene Incorrect, 1 attempt remaining The systematic name of this compound classifies the -OR group as a substituent of the hydrocarbon, which is considered the principal functional group. The ether substituent is named with the suffix 'oxy'. The general format for the systematic name of a hydrocarbon is: [prefix/substituent] + [parent] + [functional group suffix] Substituents are listed in alphabetical order. Molecules with a chiral center will indicate the absolute configuration at the beginning of its name with the R and S notation.arrow_forward5. Compressibility (6 points total). The isothermal compressibility is a measure of how hard/easy it is to compress an object (how squishy is it?) at constant temperature. It is др defined as Br=-()=-(200²)T' (a) You might wonder why there is a negative sign in this formula. What does it mean when this quantity is positive and what does it mean when this quantity is negative? (b) Derive the formula for the isothermal compressibility of an ideal gas (it is very simple!) (c) Explain under what conditions for the ideal gas the compressibility is higher or lower, and why that makes sense.arrow_forward
- 19. (3 pts) in Chapter 7 we will see a reaction of halocyclohexanes that requires that the halogen occupy an axial position with this in mind, would you expect cis-1-bromo-3-methylcyclohexane or trans-1-bromo-3-methylcyclohexane to be more reactive in this reaction? Briefly explain your choice using structures to support your answer. Mere-eries-cecleone) The tran-i-browse-3-methylcyclohexionearrow_forwardPlease help me calculate the undiluted samples ppm concentration. My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve. Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4arrow_forwardProvide an IUPAC name for each of the compounds shown. (Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to commas, dashes, etc.) H₁₂C C(CH3)3 C=C H3C CH3 CH3CH2CH CI CH3 Submit Answer Retry Entire Group 2 more group attempts remaining Previous Nextarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY