
EBK ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260475685
Author: SMITH
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 67P
(a) Draw all products formed by treatment of each dibromide (A and B) with one equivalent of
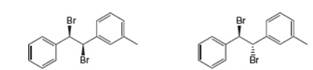
A B
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the battery:Pt | H2 (g) | H+ (aq) | Fe2+ (aq) | FeIndicate the cathode and anode.
Write the equations that occur when the electrode Pb (s) | PbI2 (s) | KI (ac) in a galvanic cell. a) It functions as a positive electrode b) It functions as a negative electrode c) What is the ion with respect to which this electrode is reversible?
State the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY
Ch. 8.1 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8.2 - Problem 8.2 Classify each alkene in the following...Ch. 8.2 - Prob. 3PCh. 8.2 - Prob. 4PCh. 8.2 - Problem 8.5 Label each pair of alkenes as...Ch. 8.2 - Problem 8.6 Which alkene in each pair is more...Ch. 8.2 - Problem 8.7 Several factors can affect alkene...Ch. 8.4 - Prob. 8PCh. 8.4 - Prob. 9PCh. 8.4 - Prob. 10P
Ch. 8.4 - Prob. 11PCh. 8.5 - Problem 8.12 What alkenes are formed from each...Ch. 8.6 - Prob. 13PCh. 8.6 - Problem 8.14 What alkenes are formed from each...Ch. 8.6 - Problem 8.15 How does each of the following...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 39PCh. 8 - Prob. 41PCh. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 56PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 63PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Sea turtles have disappeared from many regions, and one way of trying to save them is to reintroduce them to ar...
MARINE BIOLOGY
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why are normal electrode potentials also called relative electrode potentials?arrow_forwardEasily differentiate between electrochemical potential and Galvani potential.arrow_forwardConstruct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License