
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8, Problem 59P
cis-4-Bromocyclohexanol and trans-4-bromocyclohexanol form the same elimination product but a different substitution product when they react with HO-.
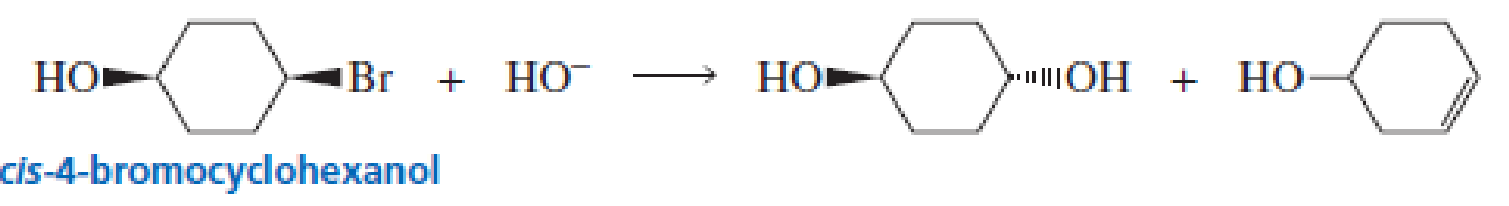
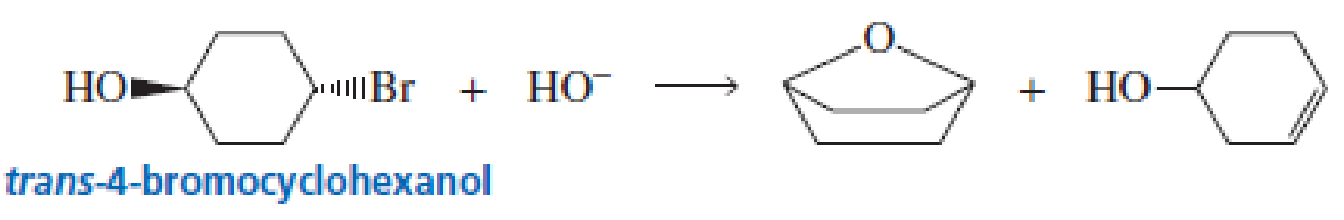
a. Why do they form the same elimination product?
b. Explain, by showing the mechanisms, why different substitution products are obtained.
c. How many stereoisomers does each of the elimination and substitution reactions form?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Given the galvanic cell whose scheme is: (-) Zn/Zn2+ ⋮⋮ Ag+/Ag (+). If we know the normal potentials E°(Zn2+/Zn) = -0.76V and E°(Ag+/Ag) = 0.799 V. Indicate the electrodes that are the anode and the cathode and calculate the E0battery.
Indicate the functions that salt bridges have in batteries.
In the battery:Pt | H2 (g) | H+ (aq) | Fe2+ (aq) | FeIndicate the cathode and anode.
Chapter 8 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 8.1 - Prob. 2PCh. 8.1 - Does increasing the energy barrier for an SN2...Ch. 8.1 - Arrange the following alkyl bromides in order from...Ch. 8.2 - Prob. 7PCh. 8.2 - Which reaction in each of the following pairs...Ch. 8.2 - Prob. 9PCh. 8.2 - Prob. 11PCh. 8.3 - Draw the substitution products that will be formed...Ch. 8.4 - Arrange the following alkyl halides in order from...Ch. 8.5 - Prob. 14P
Ch. 8.5 - Which of the following reactions will go faster if...Ch. 8.6 - After a proton is removed from the OH group, which...Ch. 8.6 - Draw the product of each of the following...Ch. 8.9 - Prob. 20PCh. 8.9 - Prob. 21PCh. 8.11 - Why do the SN1/E1 reactions of tertiary alkyl...Ch. 8.11 - Prob. 23PCh. 8.11 - Prob. 24PCh. 8.12 - Prob. 25PCh. 8.12 - Prob. 26PCh. 8.12 - Prob. 27PCh. 8.12 - a In which solvent would tert-butyl bromide...Ch. 8.13 - What would be the best way to prepare the...Ch. 8 - Methoxychlor is an insecticide that was intended...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Explain how the following changes would affect the...Ch. 8 - Prob. 38PCh. 8 - Draw the major product obtained when each of the...Ch. 8 - Which alkyl halide in Problem 39 can undergo an El...Ch. 8 - Prob. 42PCh. 8 - Prob. 43PCh. 8 - Prob. 44PCh. 8 - Prob. 45PCh. 8 - Starting with bromocyclohexane, how could the...Ch. 8 - Prob. 48PCh. 8 - Fill in the blanks in the following chemical...Ch. 8 - For each of the following alkyl halides, indicate...Ch. 8 - Prob. 51PCh. 8 - a. Explain why 1-bromo-2,2-dimethylpropane has...Ch. 8 - An ether can be prepared by an SN2 reaction of an...Ch. 8 - Give two sets of reactants (each set including an...Ch. 8 - Show how the following compounds could be...Ch. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Draw the structures of the products obtained from...Ch. 8 - cis-4-Bromocyclohexanol and...Ch. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - In which solventethanol or diethyl etherwould the...Ch. 8 - The pKa of acetic acid in water is 4.76. What...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the equations that occur when the electrode Pb (s) | PbI2 (s) | KI (ac) in a galvanic cell. a) It functions as a positive electrode b) It functions as a negative electrode c) What is the ion with respect to which this electrode is reversible?arrow_forwardState the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.arrow_forwardWhy are normal electrode potentials also called relative electrode potentials?arrow_forward
- Easily differentiate between electrochemical potential and Galvani potential.arrow_forwardConstruct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forwardplease help with hwarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY