
ORGANIC CHEMISTRY II LAB MANUAL>CUSTOM<
9th Edition
ISBN: 9780534261641
Author: SIMEK
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7.16, Problem 7.32P
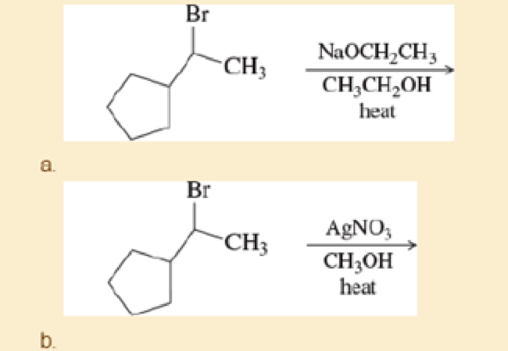
Predict the major and minor elimination products of the following proposed reactions (ignoring any possible substitutions for now). In each case, explain whether you expect the mechanism of the elimination to be E1 or E2.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Help w c!
Can someone help me understand this?
help w d!
Chapter 7 Solutions
ORGANIC CHEMISTRY II LAB MANUAL>CUSTOM<
Ch. 7.3A - Prob. 7.1PCh. 7.3A - Prob. 7.2PCh. 7.3B - Draw five more compounds of formula C4H6NOC1.Ch. 7.3B - For each of the following molecular formulas,...Ch. 7.4 - Give the systematic (IUPAC) names of the following...Ch. 7.5B - The following names are all incorrect. Draw the...Ch. 7.5B - Prob. 7.8PCh. 7.5B - a. How many stereogcmc double bonds are in...Ch. 7.6 - Teflon-coated frying pans routinely endure...Ch. 7.7B - Prob. 7.11P
Ch. 7.8B - Use the data in Table7-2 to predict the energy...Ch. 7.8C - Prob. 7.13PCh. 7.8E - Explain why each of the following alkenes is...Ch. 7.8F - Prob. 7.15PCh. 7.10 - Prob. 7.16PCh. 7.10A - SN1 substitution and E1 elimination frequently...Ch. 7.10C - Prob. 7.18PCh. 7.10C - Prob. 7.19PCh. 7.10C - Prob. 7.20PCh. 7.11 - Prob. 7.21PCh. 7.11 - Prob. 7.22PCh. 7.12 - Prob. 7.23PCh. 7.12 - Prob. 7.24PCh. 7.13 - Prob. 7.25PCh. 7.14B - Prob. 7.26PCh. 7.14B - Make models of the blowing compounds, and predict...Ch. 7.15 - Prob. 7.28PCh. 7.15 - Prob. 7.29PCh. 7.15 - Prob. 7.30PCh. 7.15 - Prob. 7.31PCh. 7.16 - Predict the major and minor elimination products...Ch. 7.17B - Predict the products and mechanisms of the...Ch. 7.18 - Propose mechanisms for the following reactions.Ch. 7.18 - Prob. 7.35PCh. 7.19B - The dehydrogenation of butane to trans-but-2-ene...Ch. 7.19B - Prob. 7.37PCh. 7.19B - Prob. 7.38PCh. 7.19B - Prob. 7.39PCh. 7 - Prob. 7.40SPCh. 7 - Prob. 7.41SPCh. 7 - Prob. 7.42SPCh. 7 - Prob. 7.43SPCh. 7 - Prob. 7.44SPCh. 7 - Prob. 7.45SPCh. 7 - Prob. 7.46SPCh. 7 - The energy difference between cis- and...Ch. 7 - Prob. 7.48SPCh. 7 - Prob. 7.49SPCh. 7 - Prob. 7.50SPCh. 7 - What halides would undergo E2 dehydrohalogenation...Ch. 7 - Prob. 7.52SPCh. 7 - Prob. 7.53SPCh. 7 - Write a balanced equation for each reaction,...Ch. 7 - Prob. 7.55SPCh. 7 - Using cyclohexane as your starting material, show...Ch. 7 - Show how you would prepare cyclopentene from each...Ch. 7 - Prob. 7.58SPCh. 7 - E1 eliminations of alkyl halides are rarely useful...Ch. 7 - Prob. 7.60SPCh. 7 - Propose mechanisms for the following reactions....Ch. 7 - Prob. 7.62SPCh. 7 - Prob. 7.63SPCh. 7 - Prob. 7.64SPCh. 7 - Prob. 7.65SPCh. 7 - Prob. 7.66SPCh. 7 - Prob. 7.67SPCh. 7 - Prob. 7.68SPCh. 7 - Prob. 7.69SPCh. 7 - Explain the dramatic difference in rotational...Ch. 7 - One of the following dichloronorbornanes undergoes...Ch. 7 - A graduate student wanted to make...Ch. 7 - Prob. 7.73SPCh. 7 - Prob. 7.74SPCh. 7 - Prob. 7.75SPCh. 7 - Prob. 7.76SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forwardC. Ν Harrow_forwarda. H3C. N H3C CH3 HCNarrow_forward
- ол 2. восцапан (46:00) Curtius rearrangment 1. NaN3, heat -OHarrow_forwardQuestion 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forward
- Indicate what the Luther equation is used for?arrow_forwardIndicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License