
Bundle: Chemistry & Chemical Reactivity, Loose-Leaf Version, 9th + OWLv2, 4 terms (24 Months) Printed Access Card
9th Edition
ISBN: 9781305367425
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 77SCQ
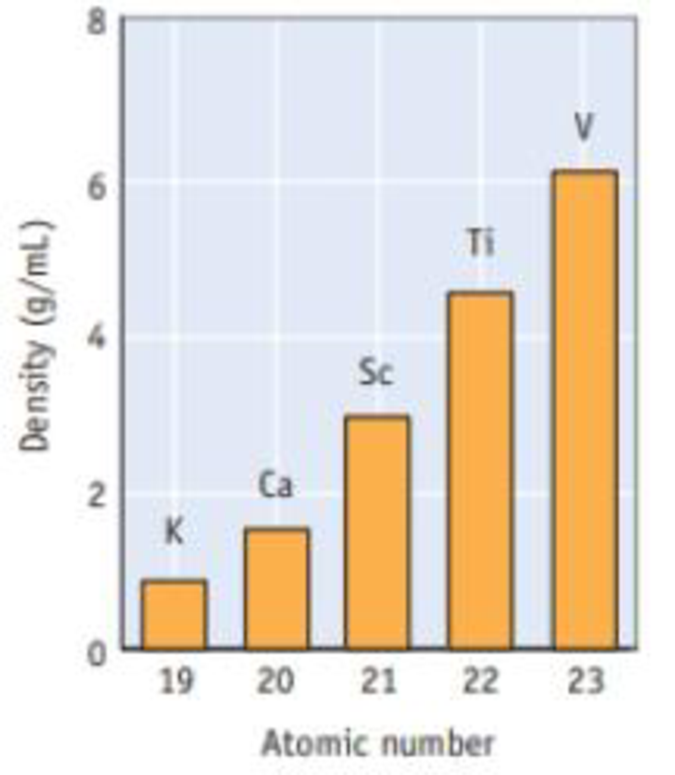
Using your knowledge of the trends in element sizes on going across the periodic table, explain briefly why the density of the elements increases from K through V.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the reaction
hand written please
Predict the major products of this organic reaction:
HBr (1 equiv)
cold
?
Some important notes:
• Draw the major product, or products, of this reaction in the drawing area below.
• You can draw the products in any arrangement you like.
• Pay careful attention to the reaction conditions, and only include the major products.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers.
• Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions.
dm
Re
Explanation
Check
©2025 McGraw Hill LLC. All Rights Reserved. Term
b) Use curved arrows to show the reaction of the radical with hydrogen bromide.
Br:
Br H
..
Answer Bank
Chapter 7 Solutions
Bundle: Chemistry & Chemical Reactivity, Loose-Leaf Version, 9th + OWLv2, 4 terms (24 Months) Printed Access Card
Ch. 7.1 - How many electrons can be accommodated in the n =...Ch. 7.1 - Prob. 2RCCh. 7.2 - Based on the Aufbau principle and the n + rule,...Ch. 7.3 - (a) What element has the configuration...Ch. 7.3 - Write one possible set of quantum numbers for the...Ch. 7.3 - Using the periodic table and without looking at...Ch. 7.3 - 1. What is the electron configuration of selenium...Ch. 7.3 - 2. Based on electron configurations, which of the...Ch. 7.4 - Prob. 1CYUCh. 7.4 - Prob. 1RC
Ch. 7.4 - Prob. 2RCCh. 7.4 - Which of the following species is most...Ch. 7.5 - Without looking at the figures for the periodic...Ch. 7.5 - What is the trend in sizes of the ions K+, S2, and...Ch. 7.5 - Prob. 2RCCh. 7.6 - Give the electron configurations for iron and the...Ch. 7.6 - Prob. 2QCh. 7.6 - Prob. 3QCh. 7.6 - Prob. 4QCh. 7.6 - Prob. 1RCCh. 7.6 - Prob. 2RCCh. 7.6 - The most common oxidation state of a rare earth...Ch. 7.6 - Prob. 6QCh. 7.6 - Prob. 7QCh. 7.6 - Use the atomic radii of scandium, yttrium,...Ch. 7.6 - Prob. 9QCh. 7.6 - Prob. 10QCh. 7 - Write the electron configurations for P and CI...Ch. 7 - Write the electron configurations for Mg and Ar...Ch. 7 - Using spdf notation, write the electron...Ch. 7 - Using spdf notation, give the electron...Ch. 7 - Prob. 5PSCh. 7 - Prob. 6PSCh. 7 - Use noble gas and spdf notations to depict...Ch. 7 - The lanthanides, once called the rare earth...Ch. 7 - Prob. 9PSCh. 7 - Prob. 10PSCh. 7 - What is the maximum number of electrons that can...Ch. 7 - What is the maximum number of electrons that can...Ch. 7 - Depict the electron configuration for magnesium...Ch. 7 - Depict the electron configuration for phosphorus...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Using orbital box diagrams, depict an electron...Ch. 7 - Prob. 18PSCh. 7 - Prob. 19PSCh. 7 - Using orbital box diagrams and noble gas notation,...Ch. 7 - Manganese is found as MnO2 in deep ocean deposits....Ch. 7 - One compound found in alkaline batteries is NiOOH,...Ch. 7 - Prob. 23PSCh. 7 - Arrange the following elements in order of...Ch. 7 - Prob. 25PSCh. 7 - Prob. 26PSCh. 7 - Which of the following groups of elements is...Ch. 7 - Arrange the following atoms in order of increasing...Ch. 7 - Compare the elements Na, Mg, O, and P. (a) Which...Ch. 7 - Compare the elements B. Al, C, and Si. (a) Which...Ch. 7 - Explain each answer briefly. (a) Place the...Ch. 7 - Explain each answer briefly. (a) Rank the...Ch. 7 - Identify the element that corresponds to each of...Ch. 7 - Identify the element that corresponds to each of...Ch. 7 - Explain why the photoelectron spectra of hydrogen...Ch. 7 - Sketch the major features (number of peaks and...Ch. 7 - These questions are not designated as to type or...Ch. 7 - The deep blue color of sapphires comes from the...Ch. 7 - Using an orbital box diagram and noble gas...Ch. 7 - Prob. 40GQCh. 7 - Prob. 41GQCh. 7 - Prob. 42GQCh. 7 - Which of the following is not an allowable set of...Ch. 7 - A possible excited state for the H atom has an...Ch. 7 - The magnet in the following photo is made from...Ch. 7 - Name the element corresponding to each...Ch. 7 - Arrange the following atoms in order of increasing...Ch. 7 - Prob. 48GQCh. 7 - Answer the questions below about the elements A...Ch. 7 - Answer (he following questions about the elements...Ch. 7 - Which of the following ions are unlikely to be...Ch. 7 - Prob. 52GQCh. 7 - Answer each of the following questions: (a) Of the...Ch. 7 - Prob. 54GQCh. 7 - Prob. 55GQCh. 7 - Two elements in the second transition series (Y...Ch. 7 - Prob. 57GQCh. 7 - The configuration of an element is given here. (a)...Ch. 7 - Answer the questions below about the elements A...Ch. 7 - Answer the questions below concerning ground state...Ch. 7 - Nickel(II) formate [Ni(HCO2)2] is widely used as a...Ch. 7 - Spinets are solids with the general formula M2+...Ch. 7 - The following questions use concepts from this and...Ch. 7 - Which ions in the following list are not likely to...Ch. 7 - Answer the following questions about first...Ch. 7 - The ionization of the hydrogen atom can be...Ch. 7 - Compare the configurations below with two...Ch. 7 - Prob. 68SCQCh. 7 - Write electron configurations to show the first...Ch. 7 - Prob. 70SCQCh. 7 - (a) Explain why the sizes of atoms change when...Ch. 7 - Which of the following elements has the greatest...Ch. 7 - Prob. 73SCQCh. 7 - Prob. 74SCQCh. 7 - The energies of the orbitals in many elements have...Ch. 7 - The ionization energies for the removal of the...Ch. 7 - Using your knowledge of the trends in element...Ch. 7 - Prob. 78SCQCh. 7 - Prob. 79SCQCh. 7 - Prob. 80SCQCh. 7 - Thionyl chloride. SOCl2, is an important...Ch. 7 - Prob. 82SCQCh. 7 - Slaters rules are a way to estimate the effective...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forward
- Primary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forwardFormulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºCarrow_forwardComplete the reaction hand written pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY