
a.
Interpretation: The geometry of
Concept Introduction:
Hybridization: It relates to the mixing of atomic orbitals into new hybrid orbitals that have varied energies and shapes. It is appropriate for the pairing of the electrons for forming
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
a.
Answer to Problem 7.65PAE
Solution: The geometry of
Explanation of Solution
The electronic configuration of I is
Structure of
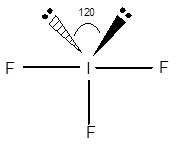
The geometry of
b.
Interpretation:
The geometry of
Concept Introduction
Hybridization: It relates to the mixing of atomic orbitals into new hybrid orbitals that have varied energies and shapes. It is appropriate for the pairing of the electrons for forming chemical bonds in the Valence Bond Theory. We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory according to which the atoms take such a position that there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp.
b.
Answer to Problem 7.65PAE
Solution:
The geometry of
Explanation of Solution
The electronic configuration of Cl is
Structure of
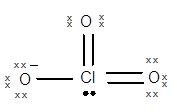
The geometry of
c.
Interpretation:
The geometry of
Concept Introduction
Hybridization: It relates to the mixing of atomic orbitals into new hybrid orbitals that have varied energies and shapes. It is appropriate for the pairing of the electrons for forming chemical bonds in the Valence Bond Theory. We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory according to which the atoms take such a position that there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
c.
Answer to Problem 7.65PAE
Solution:
The geometry of
is trigonal pyramidal as the hybridization of I is sp3d
Explanation of Solution
The electronic configuration of Tellurium is
Structure of
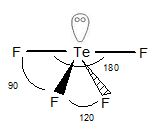
The geometry of
d.
Interpretation:
The geometry of
Concept Introduction:
Hybridization: It relates to the mixing of atomic orbitals into new hybrid orbitals that have varied energies and shapes. It is appropriate for the pairing of the electrons for forming chemical bonds in the Valence Bond Theory. We can predict the shape of a particular molecule by the knowledge of their atomic numbers and VSEPR theory according to which the atoms take such a position that there is a minimum possible repulsion between the bonded atoms and the lone pair of electrons if any.
The main concept behind this theory is that the electron pairs are always present in the outermost shell i.e. valence shell of an atom of a molecule and they repel each other due to which they try to attain the best possible position so that the value of their repulsion is the least. Hence, the electrons occupy such positions around the atom that reduces their repulsion and provides a molecule to their shape.
Here the electrons that take part in the bonding of a molecule are known as the bonding pair and the electrons that do not take part in the bonding are known as the lone pairs. The bond pairs are in the influence of the two bonding atoms whereas the lone pairs are in the influence of only of the atom.
Due to the presence of lone pairs, there is more space occupied between the atoms of the molecules. Now they suffer the repulsion between the lone pair-lone pair and bond pair-lone pair. Their repulsion can be represented as:-
lp-lp>lp-bp>bp-bp
d.
Answer to Problem 7.65PAE
Solution:
The geometry of
Explanation of Solution
The electronic configuration of Tellurium is
Structure of
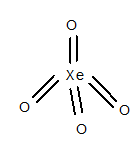
The geometry of
Want to see more full solutions like this?
Chapter 7 Solutions
EBK CHEMISTRY FOR ENGINEERING STUDENTS,
- Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.arrow_forwardHOCH, H HO CH-OH OH H OH 11 CH₂OH F II OH H H 0 + H OHarrow_forwardDraw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

