
Concept explainers
(a)
Interpretation:
It is considered that the balloon A can expand or contract. The balloon A consist ten particles of gas which is shown below. The diagram for the volume of A is to be drawn when the volume is halved and the temperature and the number of particles remain same.
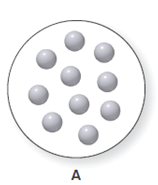
Concept Introduction:
The pressure is defined as the force which is exerted by the substance on another substance at per unit area. It is also defined as the force that is exerted by the particles of the gas on the wall of container.
(b)
Interpretation:
It is considered that the balloon A can expand or contract. The balloon A consist ten particles of gas which is shown below. The diagram for the volume of A is to be drawn when the pressure is doubled and the temperature and the number of particles remain same.
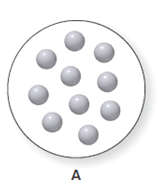
Concept Introduction:
According to Boyle's law, the change in volume results in the change in pressure at constant temperature and at constant mol of gas. It means that the pressure of sample is inversely proportional to the volume of the sample. This relation is represented as follows −
(c)
Interpretation:
It is considered that the balloon A can expand or contract. The balloon A consist ten particles of gas which is shown below. The diagram for the volume of A is to be drawn when the temperature is increased and the pressure and the number of particles remain the same.
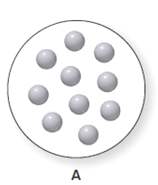
Concept Introduction:
According to Charles law, volume of gas is directly proportional to the temperature at constant pressure and at constant mol of gas which means increment in volume results in the increased value of temperature. This relation is represented as-
(d)
Interpretation:
It is considered that the balloon A can expand or contract. The balloon A consist ten particles of gas which is shown below. The diagram for the volume of A is to be drawn when the number of particles is doubled and the pressure and the temperature remain same.
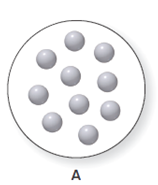
Concept Introduction:
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- Steps and explanations pleasearrow_forwardUse diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward
- Match the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forwardCan you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





