
Concept explainers
a)
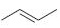
Interpretation:
The reaction of the alkene, 2-butene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, 2-butene, with a proton to give a carbocation and also to give the structure of the carbocation.
b)
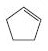
Interpretation:
The reaction of the alkene, cyclopentene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in alkenes is nucleophilic. The π electrons can be donated to a proton. By donating the π electrons one of the carbons in the double bond forms a new C-H bond while the other carbon gets a positive charge resulting in a carbocation.
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, cyclopentene, with a proton to give a carbocation and also to give the structure of the carbocation.
c)
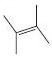
Interpretation:
The reaction of the alkene, 2,3-dimethyl-2-butene, with a proton to give a carbocation is to be shown using curved arrows. The structure of the carbocation formed as also to be given.
Concept introduction:
The double bond in alkenes is nucleophilic. The π electrons can be donated to a proton. By donating the π electrons one of the carbons in the double bond forms a new C-H bond while the other carbon gets a positive charge resulting in a carbocation.
Curved arrows start from a nucleophilic source (neutral or negatively charged) and end in an electrophilic sink (neutral or positively charged). During the flow of the electrons the octet rule must be maintained both in the source and sink.
To show:
Using curved arrows the reaction of the alkene, 2,3-dimethyl-2-butene, with a proton to give a carbocation and also to give the structure of the carbocation.
Trending nowThis is a popular solution!

Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- Complete the reaction. Not the mechanism.arrow_forwardDraw the mechanism using the arrows on conventions, including all formal charges and correct arrows. If stereochemical distinction can be made they should be included in the structure of the products.arrow_forwardDraw the epoxide formed when the following alkene is treated with mCPBA. Click the "draw structure" button to launch the drawing utility. draw structure ...arrow_forward
- Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation Check CF3 (Choose one) OH (Choose one) H (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardIdentifying electron-donating and electron-withdrawing effects For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density CF3 O donating O donating O electron-rich O withdrawing withdrawing O no inductive effects O no resonance effects O electron-deficient O similar to benzene OCH3 Explanation Check O donating O donating ○ withdrawing withdrawing O no inductive effects no resonance effects electron-rich electron-deficient O similar to benzene Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardThe acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forward
- CUE COLUMN NOTES (A. Determine Stereoisomers it has ⑤ Identify any meso B compounds cl Br cl -c-c-c-c-¿- 1 CI C- | 2,4-Dichloro-3-bromopentanearrow_forwardThe acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forwardWhat does the phrase 'fit for purpose' mean in relation to analytical chemistry? Please provide examples too.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
