
a)
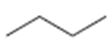
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of n-butane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of n-butane.
b)
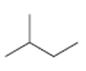
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of 2-methylbutane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro alkanes as product.
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of 2-methylbutane.
c)
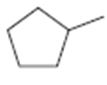
Interpretation:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of methylcyclopentane are to be drawn.
Concept introduction:
In radical chlorination reactions, if all the hydrogens present in the alkane are of same kind then it is possible to get a single monochloro product. If the alkane has hydrogens of different kinds then hydrogens belonging to all kinds will be substituted by chlorine resulting in a mixture of monochloro alkanes as product.
To draw:
The structures of different monochloro products, without considering their stereochemistry, obtainable by the radical chlorination of methylcyclopentane.
Trending nowThis is a popular solution!

Chapter 6 Solutions
EP ORGANIC CHEMISTRY,24 MONTH-OWLV2
- Problem 6-29 Identify the functional groups in the following molecules, and show the polarity of each: (a) CH3CH2C=N CH, CH, COCH (c) CH3CCH2COCH3 NH2 (e) OCH3 (b) (d) O Problem 6-30 Identify the following reactions as additions, eliminations, substitutions, or rearrangements: (a) CH3CH2Br + NaCN CH3CH2CN ( + NaBr) Acid -OH (+ H2O) catalyst (b) + (c) Heat NO2 Light + 02N-NO2 (+ HNO2) (d)arrow_forwardPredict the organic product of Y that is formed in the reaction below, and draw the skeletal ("line") structures of the missing organic product. Please include all steps & drawings & explanations.arrow_forwardPlease choose the best reagents to complete the following reactionarrow_forward
- Problem 6-17 Look at the following energy diagram: Energy Reaction progress (a) Is AG for the reaction positive or negative? Label it on the diagram. (b) How many steps are involved in the reaction? (c) How many transition states are there? Label them on the diagram. Problem 6-19 What is the difference between a transition state and an intermediate? Problem 6-21 Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall AG°, transition states, and intermediate. Is AG° positive or negative? Problem 6-23 Draw an energy diagram for a reaction with Keq = 1. What is the value of AG° in this reaction?arrow_forwardProblem 6-37 Draw the different monochlorinated constitutional isomers you would obtain by the radical chlorination of the following compounds. (b) (c) Problem 6-39 Show the structure of the carbocation that would result when each of the following alkenes reacts with an acid, H+. (a) (b) (c)arrow_forwardPlease draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic side productarrow_forward
- predict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forwardIs (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


