
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781266633973
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.3, Problem 5P
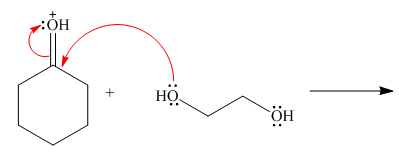
Follow the curved arrows and draw the products of the following reaction.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
can you help me solve and highlight these hw
can you please help me draw these structures for HW
can you please help me draw these two structures , it is homework
Chapter 6 Solutions
ORGANIC CHEMISTRY
Ch. 6.2 - Prob. 2PCh. 6.3 - Problem 6.3 By taking into account...Ch. 6.3 - Problem 6.4 Use curved arrows to show the movement...Ch. 6.3 - Problem 6.5 Follow the curved arrows and draw the...Ch. 6.4 - Prob. 6PCh. 6.4 - Problem 6.7 Use the values in Table 6.2 to...Ch. 6.4 - Prob. 8PCh. 6.5 - aWhich Keq corresponds to a negative value of G,...Ch. 6.5 - Given each of the following values, is the...Ch. 6.5 - Given each of the following values, is the...
Ch. 6.5 - The equilibrium constant for the conversion of the...Ch. 6.6 - Prob. 13PCh. 6.6 - For a reaction with H=40kJ/mol, decide which of...Ch. 6.6 - For a reaction with H=20kJ/mol, decide which of...Ch. 6.7 - Draw an energy diagram for a reaction in which the...Ch. 6.7 - Prob. 17PCh. 6.7 - Prob. 18PCh. 6.8 - Problem 6.19 Consider the following energy...Ch. 6.8 - Draw an energy diagram for a two-step reaction,...Ch. 6.9 - Which value if any corresponds to a faster...Ch. 6.9 - Prob. 22PCh. 6.9 - Problem 6.23 For each rate equation, what effect...Ch. 6.9 - Prob. 24PCh. 6.10 - Identify the catalyst in each equation. a....Ch. 6 - Draw the products of homolysis or heterolysis of...Ch. 6 - Explain why the bond dissociation energy for bond...Ch. 6 - Classify each transformation as substitution,...Ch. 6 - Prob. 29PCh. 6 - 6.31 (a) Add curved arrows for each step to show...Ch. 6 - Prob. 35PCh. 6 - 6.39. a. Which value corresponds to a negative...Ch. 6 - Prob. 40PCh. 6 - For which of the following reaction is S a...Ch. 6 - Prob. 42PCh. 6 - Prob. 43PCh. 6 - 6.44 Consider the following reaction: .
Use curved...Ch. 6 - Prob. 45PCh. 6 - 6.50 The conversion of acetyl chloride to methyl...Ch. 6 - Prob. 50PCh. 6 - Prob. 53P
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please help with synthesisarrow_forward10. Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the following compound. In order to recieve full credit, you MUST SHOW YOUR WORK! H₂N CI OH CI カー 11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign R/S configurations for all stereoisomers and indicate the relationship between each as enantiomer, diastereomer, or meso. NH2 H HNH, -18arrow_forwardb) 8. Indicate whether the following carbocation rearrangements are likely to occur Please explain your rational using 10 words or less not likely to occur • The double bond is still in the Same position + Likely to oc occur WHY? -3 H3C Brave Chair Conformers. Draw the chair conformer of the following substituted cyclohexane. Peform a RING FLIP and indicate the most stable conformation and briefly explain why using 20 words or less. CI 2 -cobs ?? MUST INDICATE H -2 -2 Br EQ Cl OR AT Br H& most stable WHY? - 4arrow_forward
- CH 12 Conformational Analysis. Draw all 6 conformers (one above each letter) of the compound below looking down the indicated bond. Write the letter of the conformer with the HIGHEST and LOWEST in energies on the lines provided. NOTE: Conformer A MUST be the specific conformer of the structure as drawn below -4 NOT HOH OH 3 Conformer A: Br OH A Samo Br H 04 Br H H3 CH₂ H anti stagere Br CH clipsed H Brott H IV H MISSING 2 -2 B C D E F X 6 Conformer with HIGHEST ENERGY: 13. (1 structure LOWEST ENERGY: Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the corresponding to this name. HINT: Do not forget to indicate stereochemistry when applicable. a) ८८ 2 "Br {t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa Parent name (noname) 4 Bromo Sub = 2-methylethyl-4 Bromo nonane b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane # -2 -2arrow_forwardin the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forward
- Molecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forwardIndicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY