
EBK INTRO.CHEMISTRY (NASTA EDITION)
9th Edition
ISBN: 9781337678032
Author: ZUMDAHL
Publisher: CENGAGE CO
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 69AP
When solid red phosphorus, 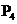 is burned in air, the phosphorus combines with oxygen, producing a choking cloud of tetraphosphorus decoxide. Write the unbalanced chemical equation for this reaction.
is burned in air, the phosphorus combines with oxygen, producing a choking cloud of tetraphosphorus decoxide. Write the unbalanced chemical equation for this reaction.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
An einstein is the amount of energy needed to dissociate 1 mole of a substance. If we have 0.58 moles, do we need 0.58 einsteins to dissociate that substance?
If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.Data: Energy of each photon: 0.7835x10-18 J.
If the energy absorbed per mole of gas is 480 kJ mol-1, indicate the number of Einsteins per mole.
Chapter 6 Solutions
EBK INTRO.CHEMISTRY (NASTA EDITION)
Ch. 6.2 - Prob. 6.1SCCh. 6.3 - Prob. 1CTCh. 6.3 - One part of the problem-solving strategy for...Ch. 6.3 - Prob. 6.2SCCh. 6.3 - Prob. 6.3SCCh. 6 - The following are actual student responses to the...Ch. 6 - Prob. 2ALQCh. 6 - Given the equation for the reaction:N2+H2NH3 ,...Ch. 6 - Prob. 4ALQCh. 6 - Can the subscripts in a chemical formula be...
Ch. 6 - Prob. 6ALQCh. 6 - Changing the subscripts of chemicals can...Ch. 6 - Table 6.1 lists some clues that a chemical...Ch. 6 - Use molecular-level drawings to show the...Ch. 6 - It is stated in Section 6.3 of the text that to...Ch. 6 - Prob. 11ALQCh. 6 - Consider the generic chemical equationaA+bBcC+dD...Ch. 6 - Prob. 13ALQCh. 6 - Which of the following correctly describes the...Ch. 6 - Which of the following correctly balances the...Ch. 6 - The reaction of an element X() with element Y() is...Ch. 6 - Prob. 1QAPCh. 6 - Prob. 2QAPCh. 6 - Although these days many people have...Ch. 6 - Prob. 4QAPCh. 6 - You have probably had the unpleasant experience of...Ch. 6 - If you’ve ever left bread in a toaster too long,...Ch. 6 - What are the substances to theleftof the arrow in...Ch. 6 - Prob. 8QAPCh. 6 - In a chemical reaction, the total number of atoms...Ch. 6 - Prob. 10QAPCh. 6 - Prob. 11QAPCh. 6 - The notation “(l)” after a substance’s formula...Ch. 6 - A common experiment to determine the relative...Ch. 6 - A common lecture demonstration called “elephant’s...Ch. 6 - If a sample of pure hydrogen gas is ignited very...Ch. 6 - Liquid hydrazine, has been used as a fuel for...Ch. 6 - If electricity of sufficient voltage is passed...Ch. 6 - Silver oxide may be decomposed by strong heating...Ch. 6 - Elemental boron is produced in one industrial...Ch. 6 - Many over-the-counter antacid tablets are now...Ch. 6 - Phosphorus trichloride is used in the manufacture...Ch. 6 - Pure silicon, which is needed in the manufacturing...Ch. 6 - Nitrous oxide gas (systematic name: dinitrogen...Ch. 6 - Solid zinc is added to an aqueous solution...Ch. 6 - Acetylene gas (C2H2) is often used by plumbers,...Ch. 6 - The burning of high-sulfur fuels has been shown to...Ch. 6 - The Group 2 metals (Ba, Ca, Sr) can be produced in...Ch. 6 - There are fears that the protective ozone layer...Ch. 6 - Carbon tetrachloride was widely used for many...Ch. 6 - When elemental phosphorus, P4, burns in oxygen...Ch. 6 - Calcium oxide is sometimes very challenging to...Ch. 6 - Prob. 32QAPCh. 6 - The element tin often occurs in nature as the...Ch. 6 - Nitric acid, HNO3 , can be produced by reacting...Ch. 6 - When balancing chemical equations, beginning...Ch. 6 - The “Chemistry in Focus” segment The Beetle That...Ch. 6 - Balance each of the following chemical equations....Ch. 6 - Balance the equations for the reaction of...Ch. 6 - Balance each of the following chemical equations....Ch. 6 - Balance each of the following chemical equations....Ch. 6 - Balance each of the following chemical equations....Ch. 6 - Prob. 42QAPCh. 6 - Prob. 43QAPCh. 6 - Prob. 44QAPCh. 6 - Acetylene gas, C2H2 , is used in welding because...Ch. 6 - When balancing a chemical equation, which of the...Ch. 6 - Crude gunpowders often contain a mixture of...Ch. 6 - The following demonstration takes place in a...Ch. 6 - Methanol (methyl alcohol), CH3OH , is a very...Ch. 6 - The Hall process is an important method by which...Ch. 6 - Iron oxide ores, commonly a mixture of FeO and...Ch. 6 - True or false? Coefficients can be fractions when...Ch. 6 - When steel wool (iron) is heated in pure oxygen...Ch. 6 - One method of producing hydrogen peroxide is to...Ch. 6 - When elemental boron, B, is burned in oxygen gas,...Ch. 6 - A common experiment in introductory chemistry...Ch. 6 - A common demonstration in chemistry courses...Ch. 6 - Write a balanced chemical equation for the...Ch. 6 - Prob. 59APCh. 6 - Prob. 60APCh. 6 - If you had a “sour stomach,” you might try an...Ch. 6 - When iron wire is heated in the presence of...Ch. 6 - When finely divided solid sodium is dropped into a...Ch. 6 - If aqueous solutions of potassium chromate and...Ch. 6 - When hydrogen sulfide, H2S , gas is bubbled...Ch. 6 - If an electric current is passed through aqueous...Ch. 6 - When a strip of magnesium metal is heated in...Ch. 6 - Prob. 68APCh. 6 - When solid red phosphorus, is burned in air, the...Ch. 6 - When copper (II) oxide is boiled in an aqueous...Ch. 6 - When lead(II) sulfide is heated lo high...Ch. 6 - Which of the following statements about chemical...Ch. 6 - Prob. 73APCh. 6 - Prob. 74APCh. 6 - Prob. 75APCh. 6 - Using different shapes to distinguish between...Ch. 6 - Which of the following statements about chemical...Ch. 6 - Prob. 78CPCh. 6 - Balance the following chemical equations....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The quantum yield of the photochemical decay of HI is 2. Calculating the moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forwardThe quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.arrow_forward
- If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.arrow_forwardWhen propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forward
- Does Avogadro's number have units?arrow_forwardExplain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forward
- Indicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forwardIndicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
GCSE Chemistry - Differences Between Compounds, Molecules & Mixtures #3; Author: Cognito;https://www.youtube.com/watch?v=jBDr0mHyc5M;License: Standard YouTube License, CC-BY