
(a)
Interpretation:
The conversion after
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
A batch reactor is a closed system with no continuous flow of reactants entering the system or products leaving the system while the reaction takes place.
In most batch reactors, the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems the conversion X is a function of the time the reactants spend in the reactor.
(a)
Answer to Problem 6.3P
The conversion after
Explanation of Solution
The given second-order liquid phase reaction which takes place in a batch reactor is as follows.
The given specific rate constant for the reaction is
The given volume of reactor 1 is
The concentration of each reactant after the mixing is
The temperature of the batch reactor is
The given two reactors are shown below.
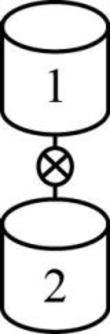
Figure 1
The concentration of the reactant is assumed to be
Where,
The expression for the integrated general concentration of the reactant before mixing the
Where,
Substitute the value of
Substitute
The expression to calculate the conversion after
Substitute
For the conversion after
Substitute
For the conversion after
Substitute
Therefore, the conversion after
(b)
Interpretation:
The conversion and concentration of each species present in the reactor 1 after
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
A batch reactor is a closed system with no continuous flow of reactants entering the system or products leaving the system while the reaction takes place.
In most batch reactors, the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems the conversion X is a function of the time the reactants spend in the reactor.
(b)
Answer to Problem 6.3P
The concentration after 10,
Explanation of Solution
The given second-order liquid phase reaction which takes place in a batch reactor is as follows.
The given specific rate constant for the reaction is
The given volume of reactor 1 is
The concentration of each reactant after the mixing is
The temperature of the batch reactor is
The given two reactors are shown below.
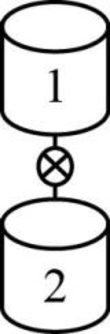
Figure 1
It is given that after 10 minutes, the species in the reactor 1 are drained out at the rate of
The solution that is left out in 10 minutes is
The total solution that is remaining in the reactor 1 is
If the solution is
So, in
The concentration of each species is calculated below given below.
Thus, the concentration of each species remains the same in
So, the concentration of each species after
The concentration of the product after
So, the concentration of each species after
The concentration of the product after
The expression to calculate the conversion after
Substitute
Substitute
For the conversion after
The concentration of the product after
Therefore, the concentration after
Substitute
Therefore, the conversion after
(c)
Interpretation:
The conversion and concentration of each species present in the reactor 2 that is filling up from reactor 1 after
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
A batch reactor is a closed system with no continuous flow of reactants entering the system or products leaving the system while the reaction takes place.
In most batch reactors, the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems the conversion X is a function of the time the reactants spend in the reactor.
(c)
Answer to Problem 6.3P
The conversion of each species present in the reactor 2 that is filling up from reactor 1 after
Explanation of Solution
The given second-order liquid phase reaction which takes place in a batch reactor is as follows.
The given specific rate constant for the reaction is
The given volume of reactor 1 is
The concentration of each reactant after the mixing is
The temperature of the batch reactor is
The given two reactors are shown below.
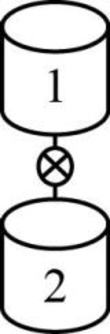
Figure 1
It is given that after 10 minutes, the species in the reactor 1 are drained out at the rate of
The solution that is left in 10 minutes is
The total solution that is remaining in the reactor 1 is
If the solution is
So, in
The concentration of each species is calculated below given below.
Thus, the concentration of each species remains the same in
So, the concentration of each species after
The concentration of the product after
At the end of 50 minutes, the volume of reactor 1 is
The concentration of the product after
At the end of 50 minutes, the volume of reactor 2 has
Therefore, the concentration after
Therefore, the conversion after
(d)
Interpretation:
The overall conversion and concentration when the contents of the reactants are mixed together at the end of
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
A batch reactor is a closed system with no continuous flow of reactants entering the system or products leaving the system while the reaction takes place.
In most batch reactors, the longer a reactant stays in the reactor, the more the reactant is converted to product until either equilibrium is reached or the reactant is exhausted. Consequently, in batch systems the conversion X is a function of the time the reactants spend in the reactor.
(d)
Answer to Problem 6.3P
The overall conversion of the two reactor after mixing the contents is
Explanation of Solution
The given second-order liquid phase reaction which takes place in a batch reactor is as follows.
The given specific rate constant for the reaction is
The given volume of reactor 1 is
The concentration of each reactant after the mixing is
The temperature of the batch reactor is
The given two reactors are shown below.
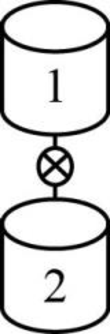
Figure 1
At the end of 50 minutes, the volume of reactor 1 is
The concentration of the product after
The number of moles of reactant are
The number of moles of the product are
At the end of 50 minutes, the volume of reactor 2 is
So, the total volume after the mixing of the species of reactor 1 and 2 is
The total moles of reactants after the mixing of the species of reactor 1 and 2 is
The total concentration of reactants after the mixing of the species of reactor 1 and 2 is
The overall conversion after mixing in reactor,
The total moles of product after the mixing of the species of reactor 1 and 2 is
The total concentration of product after the mixing of the species of reactor 1 and 2 is
The conversion,
Therefore, the overall conversion of the two reactor after mixing the contents is
(e)
Interpretation:
The application of the six ideas that is given in table on this problem is to be stated.
Concept Introduction:
The conversion, X can be defined as the moles of any species A that are reacted per mole of A fed in the reactor.
The reactors that consist of the particles of solid catalysts which are packed in the form of bed are known as fixed bed reactors.
(e)
Answer to Problem 6.3P
The application of the six ideas that is given in table on this problem is not appropriate.
Explanation of Solution
The given six ideas are as follows.
- Brainstorm ideas to ask another question or advice another calculation for the homework problem.
- Brainstorm ways to work this homework problem incorrectly.
- Brainstorm ways to make this problem highly easy or more difficult.
- Brainstorm a list of things that can be learned from working this homework problem.
- Brainstorm the reason why the calculations over predicted the conversion that measured if the reactor was kept on stream.
- The “What if…” questions are particularly effective when they are used with the living example problems in which variation in parameters to explore the problem is carried out.
These six ideas are not relevant according to the demand of the question. Thus, The application of the six ideas that is given in table on this problem cannot be possible according to the demand of the question.
Want to see more full solutions like this?
Chapter 6 Solutions
ELEMENTS OF CHEM. REACTION ENGR
- Please do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardIn the published paper, "Exergy-based Greenhouse gas metric of buildings", use the value of the Exergy Loss of Emission of carbon dioxide to evaluate the index value for 1000 occupants for 50 years building life span in kilogram per person per yeararrow_forwardA CO₂-saturated brine (ionic strength = 2.5 mol/kg, pH = 3, a_H⁺ = 0.01) flows at 2 cm/s through a horizontal tubular reactor (L = 1 m, D = 0.05 m) packed with 5 kg of olivine (Zhuravlev-BET surface area = 30 m²/g). The system operates at 90 °C and 40 bar, and external mass transfer resistance is negligible. The rate-limiting step is electron transfer at the mineral surface, governed by Marcus theory, with λ = 0.75 eV, ΔG° = –0.30 eV, and k₀ = 10⁶ s⁻¹. The Mg²⁺ activity coefficient is γ = 0.76 (from PHREEQC with Pitzer model). For Mg₂SiO₄ + 4 H⁺ → 2 Mg²⁺ + SiO₂(aq) + 2 H₂O, determine the total moles of Mg²⁺ released after 10 minutes at steady state.arrow_forward
- In baseball, batters frequently attempt to hit a ball as far as possible. However, baseballs are inelastic with an officially required "coefficient of restitution" CR ≈ 0.55 on ash wood. The coefficient of restitution of a dropped ball iswhere H is the initial drop height, h is the max. height on the rebound, and h ≈ 2πH tan δ for a homogeneous material. Assuming that a baseball is homogeneous and has a storage modulus approximately the same as that of cork (E' = 18.6 MPa), what must the value of the loss modulus E'' be so that the ball is regulation?arrow_forwardThe creep strain rate of a polymer (in “Hz”) is given by where T is the temperature and Q = 100. kJ/mol is the activation energy. How long t will it take for a rod of this polymer to extend from 10. mm to 15 mm at 100. °C?arrow_forwardCreep compliance J(t) An amorphous polymer has Tg = 100 °C. A creep modulus of 1/J = 1 GPa was measured after t₁ = 1 h at T₁ = 90 °C. Suppose that log 10 a (T) = 17.5(T-Tg) 52+(T-Tg) 1 for this material. What is the shift factor a at T = T₁ relative to the reference Tg? What is the time t₂ required to reach a modulus of 1 GPa at T2 = 80 °C? TR = T = 100 °C |J(t) = 1 GPa-1 + log a(T₁) T₁ = 75 °C T₂ = 50 °C log tr log t₁ log t log t₂ = ?arrow_forward
- A 0.45 mol/kg aqueous solution of 3-(methylamino)propylamine and 1-methylpiperazine (1:1 molar) flows at 0.5 g/s through a 2.5 m horizontal stainless steel coil (inner diameter 1.2 mm), entering at 358.15 K and 22 MPa and exiting at 20 MPa. A constant wall heat flux of 28 W is applied. Local density and isobaric heat capacity are obtained through from the Benedict–Webb–Rubin equation of state, with a 3% increase in heat capacity to account for wall sorption. Dynamic viscosity is obtained using the Green-Kubo relation with a given integral value of 2.1 × 10⁻¹⁰ Pa².s, and thermal conductivity is assumed constant. The local Nusselt number is corrected for thermal development using Nu(x) = 3.66 + (0.065 · Gz(x)^0.7) / (1 + 0.04 · Gz(x)^0.7), where Gz(x) = D · Re(x) · Pr(x) / x. Through a spatially resolved numerical integration of the 1D steady-state energy equation and determine the outlet temperature (K).arrow_forwardA pilot process is being planned to produce antibiotic P. Antibiotic P is a compound secreted by microorganism A during the stationary phase. To produce P, substrate S is required. The growth of microorganism A follows the Monod equation, with a maximum specific growth rate $\mu_m = 1 h^{-1}$ and a half-saturation constant $K_s = 700\ mg/L$.The pilot process uses a chemostat with a working volume of $1000\ L$. In this chemostat, the outflow is processed to separate microorganisms, which are then concentrated tenfold and recycled. A sterile medium containing $15\ g/L$ of substrate is supplied at a flow rate of $100\ L/h$, while the recycled flow (concentrated) contains $5\ g/L$ and is also fed into the chemostat.Microorganism A yields $0.5\ g$ of biomass per $1\ g$ of substrate consumed $(Y^M_{X/S} = 0.5\ g\ A/g\ S)$, and its death rate $(k_d)$ is negligible. Additionally, $1\ g$ of microorganism A produces $0.05\ g$ of antibiotic P per hour $(q_P = 0.05 g\ P/h\cdot g\ A)$, and $1\ g$…arrow_forwardIn the production of ethyl acetate via reactive distillation, the column operates at 5 bar with an equimolar feed (ethanol + acetic acid) at 80°C. The reaction follows: \[CH_3COOH + C_2H_5OH \rightleftharpoons CH_3COOC_2H_5 + H_2O \quad (K_{eq} = 4.2 \text{ at } 80°C)\] Given: - NRTL parameters for all binary pairs - Tray efficiency = 65% - Vapor-liquid equilibrium exhibits positive azeotrope formation Calculate the exact minimum reflux ratio required to achieve 98% ethyl acetate purity in the distillate, assuming: 1) The reaction reaches equilibrium on each tray 2) The heavy key component is waterarrow_forward
- In a multi-stage distillation column designed to separate a binary mixture of ethanol and water, the mass flow rate of the feed entering the column is \( F \), and the distillate product flow rate is \( D \). The reflux ratio \( R \) is defined as the ratio of the liquid returned to the column to the distillate flow rate. For the ideal case, where the column operates at maximum efficiency, determine the **minimum reflux ratio** \( R_{\text{min}} \) when the relative volatility \( \alpha = 1.5 \).arrow_forwardQ2: Draw the layout of a basic asynchronous 32k * 8 SRAM.arrow_forwardA distillation column with 100 kmol/h feed of 50% A and 50% B produces a distillate product with xD = 0.95 and a bottom stream with xbot = 0.04 of the more volatile species A. CMO is valid and the equilibrium data is given by y = 2.4x/1 + 1.4x a) If the feed is saturated liquid, determine the minimum reflux ratio b) If the feed is saturated vapor, determine the minimum reflux ratioarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





