
(a)
To determine: The method of conversion of
Interpretation: The method of conversion of
Concept introduction: NBS is N-bromosuccinimide. It is used as an alternative for
(a)
Answer to Problem 6.30SP
The conversion of
Explanation of Solution
The conversion of
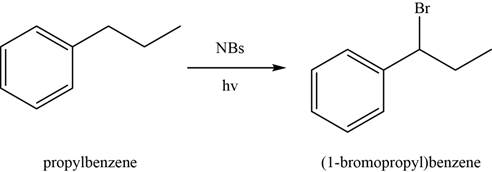
Figure 1
In the above reaction,
(b)
To determine: The method of conversion of
Interpretation: The method of conversion of
Concept introduction: NBS is N-bromosuccinimide. It is used as an alternative for
(b)
Answer to Problem 6.30SP
The conversion of
Explanation of Solution
In first step,
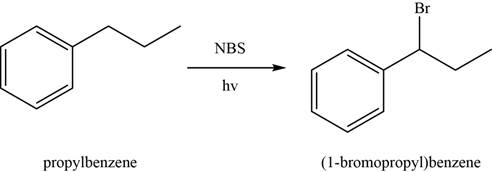
Figure 2
In the second step,
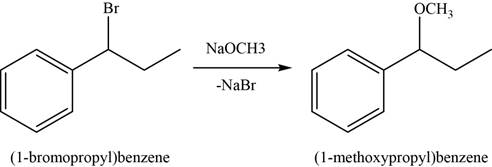
Figure 3
(c)
To determine: The method of conversion of
Interpretation: The method of conversion of
Concept introduction: NBS is N-bromosuccinimide. It is used as an alternative for
(c)
Answer to Problem 6.30SP
The conversion of
Explanation of Solution
In first step,
In the second step,
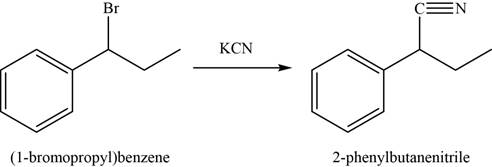
Figure 4
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry, Books a la Carte Edition (9th Edition)
- What is the missing reactant in this organic reaction? CH3-C-CH2-NH2 + R - CH3 O: 0 CH3-N-CH2-C-NH-CH2-C-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click anywhere to draw the first atom of your structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardDone 18:17 • www-awu.aleks.com Chapter 12 HW Question 24 of 39 (4 points) | Question Attempt: 1 of Unlimited ▼ 20 ✓ 21 × 22 23 24 25 26 raw the structure corresponding to each IUPAC name. Part 1 of 2 .III LTE 22 27 28 סוי 29 29 3 A skeletal structure corresponding to the IUPAC name 3-ethyl-4-methylhexane. Part 2 of 2 Click and drag to start drawing a structure. A condensed structure corresponding to the IUPAC name 2,2,4- trimethylpentane. Click anywhere to draw the first atom of your structure. Check Save For Later Submit < Х ப: G © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility : Garrow_forwardDone 18:25 www-awu.aleks.com .III LTE Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimi... Oli 23 24 25 26 27 28 29 30 Consider this structure. CH2 CH2CH2 CH2CH2CH₂ C -C. -CH2CH3 H CH Part: 0 / 3 Part 1 of 3 Give the IUPAC name of this structure. Skip Part < Check ☑ Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility ....................arrow_forward
- Calculate Ecell at 25.0 oC using the following line notation. Zn(s)|Zn+2(aq, 0.900 M)||Cu+2(aq, 0.000200 M)|Cu(s)arrow_forwardPredict the product of this organic reaction: O OH + H + OH A P + H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click and drag to start drawing a structure. X G ☐ :arrow_forward0.0994 g of oxalic acid dihydrate is titrated with 10.2 mL of potassium permanganate. Calculate the potassium permanganate concentration. Group of answer choices 0.0433 M 0.135 M 0.0309 M 0.193 Marrow_forward
- Experts...can any one help me solve these problems?arrow_forwardAccording to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardWhich Group 1 metal reacts with O2(g) to form a metal peroxide (M2O2)? Group of answer choices Li K Rb Naarrow_forward
- Which of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forwardWhich element cannot interact with hydrogen through hydrogen bonds? Group of answer choices O S Br Narrow_forwardWhich of the following statements is false regarding hydrogen gas production? Group of answer choices Steam reforming requires a catalyst. Methanol (CH3OH) can react with water using a ZnO catalyst to form H2(g). Methanol (CH3OH) can react with O2(g) using a Pd catalyst to form H2(g). The reaction between CH4(g) and H2O to form H2(g) requires a temperature of at least 700 oCarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

