
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: Structure and Properties (2nd Edition)
2nd Edition
ISBN: 9780134565613
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 2SAQ
Determine the hybridization about C in H2CO.
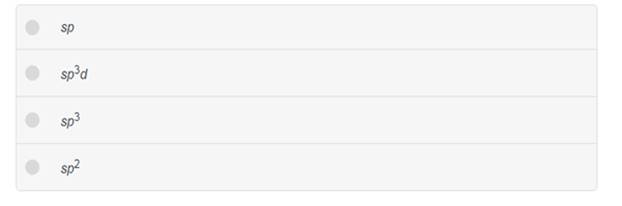
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.
Complete the reaction in the drawing area below by adding the major products to the right-hand side.
If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead.
Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule
to represent each pair of enantiomers, using line bonds at the chiral center.
More...
No reaction.
my
ㄖˋ
+
1. Na O Me
Click and drag to start
drawing a structure.
2. H
+
Predict the intermediate 1 and final product 2 of this organic reaction:
NaOMe
H+
+
1
2
H
H
work up
You can draw 1 and 2 in any arrangement you like.
Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center.
Click and drag to start drawing a structure.
X
$
dm
Chapter 6 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Chemistry: Structure and Properties (2nd Edition)
Ch. 6 - Prob. 1ECh. 6 - What is a chemical bond according to valence bond...Ch. 6 - In valence bond theory, what determines the...Ch. 6 - In valence bond theory, the interaction energy...Ch. 6 - What is hybridization? Why is hybridization...Ch. 6 - How does hybridization of the atomic orbitals in...Ch. 6 - How is the number of hybrid orbitals related to...Ch. 6 - Sketch each hybrid orbital sp sp2 sp3 sp3d sp3d2Ch. 6 - Prob. 9ECh. 6 - Name the hybridization scheme that corresponds to...
Ch. 6 - What is a chemical bond according to molecular...Ch. 6 - Explain the difference between hybrid atomic...Ch. 6 - What is a bonding molecular orbital?Ch. 6 - Prob. 14ECh. 6 - What is the role of wave interference in...Ch. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - When applying molecular orbital theory to...Ch. 6 - In molecular orbital theory, what is a nonbonding...Ch. 6 - Write a short paragraph describing chemical...Ch. 6 - The valence electron configurations of several...Ch. 6 - The valence electron configurations of several...Ch. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Prob. 29ECh. 6 - Draw orbital diagrams (boxes with arrows in them)...Ch. 6 - Which hybridization scheme allows the formation of...Ch. 6 - Which hybridization scheme allows the central atom...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Write a hybridization and bonding scheme for each...Ch. 6 - Consider the structure of the amino acid alanine...Ch. 6 - Consider the structure of the amino acid aspartic...Ch. 6 - Sketch the bonding molecular orbital that results...Ch. 6 - Sketch the antibonding molecular orbital that...Ch. 6 - Draw an MO energy diagram and predict the bond...Ch. 6 - Draw an MO energy diagram and predict the bond...Ch. 6 - Sketch the bonding and antibonding molecular...Ch. 6 - Sketch the bonding and antibonding molecular...Ch. 6 - Using the molecular orbital energy ordenng for...Ch. 6 - Using the molecular orbital energy ordering for...Ch. 6 - Apply molecular orbital theory to predict if each...Ch. 6 - Apply molecular orbital theory to predict if each...Ch. 6 - According to MO theory, which molecule or ion has...Ch. 6 - According to MO theory, which molecule or ion has...Ch. 6 - Draw an MO energy diagram for CO. (Use the energy...Ch. 6 - Draw an MO energy diagram for HCI. Predict the...Ch. 6 - For each compound, draw the Lewis structure,...Ch. 6 - For each compound, draw the Lewis structure,...Ch. 6 - Amino acids are biological compounds that link...Ch. 6 - The genetic code is based on four different bases...Ch. 6 - The structure of caffeine, present in coffee and...Ch. 6 - The structure of acetylsalicylic acid (aspirin) is...Ch. 6 - Draw a molecular orbital energy diagram for CIF....Ch. 6 - Draw Lewis structures and MO diagrams for CN+, CN,...Ch. 6 - Bromine can form compounds or ions with any number...Ch. 6 - The compound C3H4 has two double bonds. Describe...Ch. 6 - How many hybrid orbitals do we use to describe...Ch. 6 - Prob. 66ECh. 6 - In VSEPR theory, which uses the Lewis model to...Ch. 6 - The resuts of a molecular orbital calculation for...Ch. 6 - Prob. 69ECh. 6 - cis-2-Butene isomerizes (changes its structure) to...Ch. 6 - The ion CH5 + can form under very special...Ch. 6 - Neither the VSEPR model nor the hybridization...Ch. 6 - Prob. 73ECh. 6 - The most stable forms of the nonmetals in groups...Ch. 6 - Consider the bond energies of three iodine...Ch. 6 - How many atomic orbitals form a set of sp3hybrid...Ch. 6 - Have each group member pick one of these...Ch. 6 - Divide your group into two subgroups. Have one...Ch. 6 - A molecular orbital calculation for Hi results in...Ch. 6 - Determine the hybridization about 0 in CH3OH.Ch. 6 - Determine the hybridization about C in H2CO.Ch. 6 - According to the valance bond theory, which kind...Ch. 6 - Use molecular orbital theory to determine the bond...Ch. 6 - Use molecular orbital theory to predict which...Ch. 6 - Use molecular orbital theory to determine which...Ch. 6 - Which hybridization scheme occurs about nitrogen...Ch. 6 - Prob. 8SAQCh. 6 - Prob. 9SAQCh. 6 - Prob. 10SAQCh. 6 - Which type of orbitals overlap to form the sigma...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major products of this organic reaction: 1. NaH (20°C) 2. CH3Br ? Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. G Crarrow_forwardPredict the major products of this organic reaction: 1. LDA (-78°C) ? 2. Br Some notes: • Draw only the major product, or products. You can draw them in any arrangement you like. . • Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers. • If there are no products, just check the box under the drawing area. No reaction. Click and drag to start drawing a structure. Xarrow_forwardPlease draw the structuresarrow_forward
- Draw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 0 1. Eto 1. Eto- 1 2 2. MeBr 2. EtBr H3O+ A 3 You can draw the three structures in any arrangement you like. Explanation Check Click and drag to start drawing a structure.arrow_forwardDraw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forward
- What is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- > aw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 1. Eto 1. EtO¯ H3O+ 1 2 2. PrBr 2. PrBr Δ You can draw the three structures in any arrangement you like. 3 Click and drag to start drawing a structure. Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacarrow_forwardThere are various factors that affect an equilibrium. Give 3 of these factors and explain using examples andequations how an equilibrium is affected by these factors. Please remember that this is a communication question so that you are communicating your understanding of the factors that affect and equilibrium.arrow_forwardEEZE LETCHUP ID Draw the most likely conjugate base resulting from this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. Drawing く NaOCH2CH3 :0: :0: 狗arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY