
(a)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,
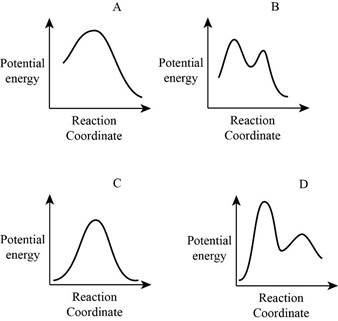
Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(b)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,
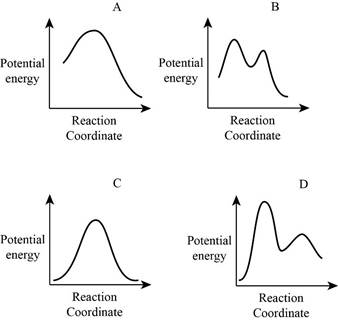
Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(c)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,
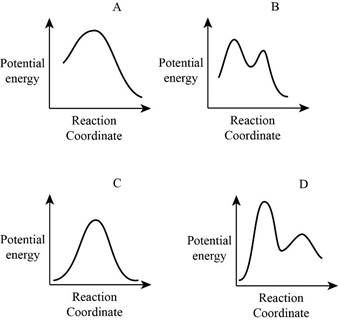
Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(d)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,
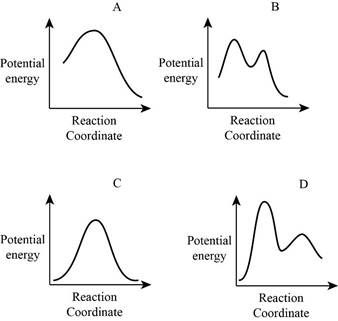
Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(e)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,

Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(f)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,

Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(g)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,

Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
(h)
Interpretation:
From the given energy diagram – step of the mechanism,
Concept introduction:
Step of the mechanism,

Mechanism of the reaction is identified by using energy profile diagram; if the reaction is completed in a single step, there is only one the hump in energy diagram. Whereas if the reaction is completed in two steps there are two humps in the energy diagram, and when the Reaction is completed in multi-step there is multi hump in the energy diagram.
Activation energy (
The activation energy (
Equilibrium Constant (
Equilibrium Constant (
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





