
Concept explainers
Determine the number of nodes along the internuclearaxis for each of the
Interpretation: The number of nodes along the internuclear axis for each of the
Concept introduction: Born-Oppenheimer approximation state that nuclei are heavier than electrons that considered fixed in space whereas the electrons move constantly around them. The Born−Oppenheimer approximation solves the electronic Schrodinger equation for
Answer to Problem 1P
The number of nodes in
Explanation of Solution
Node is point in molecular orbitals where probability to find the electron density is zero. Nodal plane is the imaginary plane where probability to find electrons is zero.
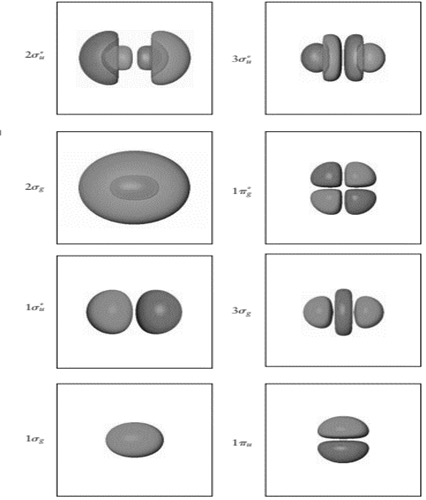
In the figure, node is the oval region. It is formed when plane white surface cut the spherical orbital into two equal halves having opposite symmetry. Also, there are six
In
In
In
In
In
In
Therefore, number of nodes in
Want to see more full solutions like this?
Chapter 6 Solutions
Student Solutions Manual for Oxtoby/Gillis/Butler's Principles of Modern Chemistry, 8th
- If the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forward
- With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forwardApply the NANSTE law to the MnO4- + 8H+ + 5e- ⇄ Mn2+ + 4H2Oarrow_forwardIn the Nernst Law, how much is RT / F?arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





