
Concept explainers
Interpretation: The structure for the exo product formed by the reaction of cyclopentadiene with maleic anhydride should be predicted.
Concept introduction:
The reaction between a diene (4π electron system) and a dienophile (2π electron system) to give a substituted cyclohexane derivative is termed as Diels Alder reaction. This reaction is a cycloaddition reaction.
Answer to Problem 1Q
The structure for the exo product formed by the reaction of cyclopentadiene with maleic anhydride is represented as follows:
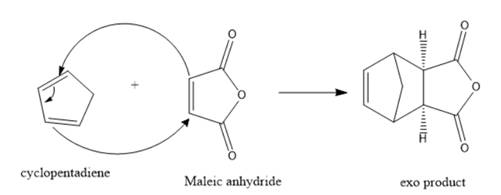
Explanation of Solution
Cyclopentadiene is a 4π electron system thus, it will be the diene. On the other hand, maleic anhydride is a 2π electron system thus, it will act as a dienophile. Therefore, the product formation for the reaction between cyclopentadiene and maleic anhydride should be represented as follows:
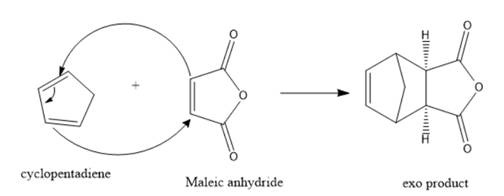
Want to see more full solutions like this?
Chapter 52 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. H3C Br .CH3 H3C NH2arrow_forwarda. OH H₂N-O -Ph H+ acyclic productarrow_forwardeks.com/aleksogi/x/sl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvTCeeBZbufuBYTI0Hz7m7D3ZS17Hd6m-HIl6n52njJN-TXdQA2X9yID-1SWQJTgnjARg30 111 States of Matter Understanding conceptual components of the enthalpy of solution 0/5 Ge A small amount of acetonitrile (CH, CN) is dissolved in a large amount of water. Imagine separating this process into the four stages sketched below. (These sketches show only a portion of the substances, so you can see the density and distribution of atoms and molecules in them.) CH,CN H₂O B 88 C Use these sketches to answer the questions in the table below. The enthalpy of solution AH is negative soln when CH3CN dissolves in water. Use this information to list the stages in order of increasing enthalpy. Would heat be absorbed or released if the system moved from Stage C to D? What force would oppose or favor the system moving from Stage C to D? Check all that apply. 1 absorbed O released neither absorbed nor released. none O ionic bonding force covalent bonding force…arrow_forward
- In a system with an anodic overpotential, the variation of ŋ as a function of the current density: 1. at low fields is linear 2. at higher fields, it follows Tafel's law Find the range of current densities for which the overpotential has the same value as when calculated for cases 1 and 2 (maximum relative difference of 5% with respect to the behavior for higher fields). To which overpotential range does this correspond? Data: 10 = 1.5 mA cm², T = 300°C, ẞ = 0.64, R = 8.314 J K 1 mol¹ and F = 96485 C mol-1.arrow_forwardIndicate 10.6 with only one significant figure.arrow_forwardIf I have 10 data points for variables x and y, when I represent y versus x I obtain a line with the equation y = mx + b. Is the slope m equal to dy/dx?arrow_forward
- The data for the potential difference of a battery and its temperature are given in the table. Calculate the entropy change in J mol-1 K-1 (indicate the formulas used).Data: F = 96485 C mol-1arrow_forwardIn a cell, the change in entropy (AS) can be calculated from the slope of the E° vs 1/T graph. The slope is equal to -AS/R, where R is the gas constant. Is this correct?arrow_forwardUsing the Arrhenius equation, it is possible to establish the relationship between the rate constant (k) of a chemical reaction and the temperature (T), in Kelvin (K), the universal gas constant (R), the pre-exponential factor (A) and the activation energy (Ea). This equation is widely applied in studies of chemical kinetics, and is also widely used to determine the activation energy of reactions. In this context, the following graph shows the variation of the rate constant with the inverse of the absolute temperature, for a given chemical reaction that obeys the Arrhenius equation. Based on the analysis of this graph and the concepts acquired about the kinetics of chemical reactions, analyze the following statements: I. The activation energy (Ea) varies with the temperature of the system. II. The activation energy (Ea) varies with the concentration of the reactants. III. The rate constant (K) varies proportionally with temperature. IV. The value of the…arrow_forward
- In an electrolytic cell, indicate the formula that relates E0 to the temperature T.arrow_forward-- 14:33 A Candidate Identification docs.google.com 11. Compound A can transform into compound B through an organic reaction. From the structures below, mark the correct one: HO A تھے۔ די HO B ○ A) Compounds A and B are isomers. B) Both have the same number of chiral carbons. C) Compound A underwent an addition reaction of Cl2 and H2O to form compound B. D) Compound A underwent a substitution reaction forming the intermediate chlorohydrin to obtain compound B. E) Compound A underwent an addition reaction of Cl2 forming the chloronium ion and then added methanol to obtain compound B. 60arrow_forward-- 14:40 A Candidate Identification docs.google.com 13. The compound 1-bromo-hex-2-ene reacts with methanol to form two products. About this reaction, mark the correct statement: OCH3 CH3OH Br OCH3 + + HBr A B A) The two products formed will have the same percentage of formation. B) Product B will be formed by SN1 substitution reaction with the formation of an allylic carbocation. C) Product A will be formed by SN1 substitution reaction with the formation of a more stable carbocation than product B. D) Product A will be formed by an SN2 substitution reaction occurring in two stages, the first with slow kinetics and the second with fast kinetics. E) The two compounds were obtained by addition reaction, with compound B having the highest percentage of formation. 57arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole



