
EBK STUDENT SOLUTIONS MANUAL TO ACCOMPA
7th Edition
ISBN: 9781119360902
Author: HYSLOP
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 73RQ
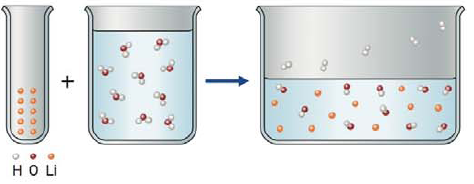
Write the balanced chemical equation for the following reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identifying the major species in weak acid or weak base equilibria
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HF is a weak acid.
2.2 mol of NaOH is added to
1.0 L of a 1.4M HF
solution.
acids:
П
bases:
Х
other: ☐
ப
acids:
0.51 mol of KOH is added to
1.0 L of a solution that is
bases:
1.3M in both HF and NaF.
other: ☐
00.
18
Ar
Using reaction free energy to predict equilibrium composition
Consider the following equilibrium:
N2O4 (g) 2NO2 (g)
AG⁰ = 5.4 kJ
Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system:
rise
Under these conditions, will the pressure of N2O4 tend to rise or fall?
x10
fall
Is it possible to reverse this tendency by adding NO2?
In other words, if you said the pressure of N2O4 will tend to rise, can that
be changed to a tendency to fall by adding NO2? Similarly, if you said the
pressure of N2O4 will tend to fall, can that be changed to a tendency to
rise by adding NO2?
If you said the tendency can be reversed in the second question, calculate
the minimum pressure of NO 2 needed to reverse it.
Round your answer to 2 significant digits.
yes
no
0.42 atm
☑
5
0/5
?
مله
Ar
Homework 13 (Ch17)
Question 4 of 4 (1 point) | Question Attempt: 2 of 2
✓ 1
✓ 2
= 3
4
Time Remaining: 4:25:54
Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction:
2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g)
Round your answer to zero decimal places.
☐ kJ
x10
☐
Subm
Check
2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce
Chapter 5 Solutions
EBK STUDENT SOLUTIONS MANUAL TO ACCOMPA
Ch. 5 - When sodium reacts with molecular oxygen, O2, the...Ch. 5 - Prob. 2PECh. 5 - The chlorite ion, ClO2, is a potent disinfectant,...Ch. 5 - Assign oxidation numbers to each atom in...Ch. 5 - Prob. 5PECh. 5 - Chlorine dioxide, ClO2, is used to kill bacteria...Ch. 5 - The following equation is nor balanced. Explain...Ch. 5 - Practice Exercise 5.8
The element technetium...Ch. 5 - Practice Exercise 5.9
Consider the following...Ch. 5 - Balance the following equation for a basic...
Ch. 5 - Write the balanced half-react ions for the...Ch. 5 - Prob. 12PECh. 5 - Suppose an aqueous mixture is prepared containing...Ch. 5 - Prob. 14PECh. 5 - Prob. 15PECh. 5 - Write a balanced equation for the combustion of...Ch. 5 - Prob. 17PECh. 5 - Prob. 18PECh. 5 - Prob. 1RQCh. 5 - Prob. 2RQCh. 5 - Prob. 3RQCh. 5 - Are the following redox reactions? Explain....Ch. 5 - If the oxidation number of nitrogen in a certain...Ch. 5 - When balancing redox reactions, which side of a...Ch. 5 - Prob. 7RQCh. 5 - What are the net charges on the left and right...Ch. 5 - In Question 5.8, which half-reaction represents...Ch. 5 - The following equation is not balanced....Ch. 5 - 5.11 What is a nonoxidizing acid? Give two...Ch. 5 - What is the strongest oxidizing agent in an...Ch. 5 - Prob. 13RQCh. 5 - Prob. 14RQCh. 5 - What is a single replacement reaction?Ch. 5 - If a metal is able to react with a solution of...Ch. 5 - Prob. 17RQCh. 5 - Prob. 18RQCh. 5 - Where in the activity series do we find the best...Ch. 5 - When manganese reacts with silver ions, is...Ch. 5 - Prob. 21RQCh. 5 - Prob. 22RQCh. 5 - Prob. 23RQCh. 5 - If one of the impurities in diesel fuel has the...Ch. 5 - Prob. 25RQCh. 5 - Prob. 26RQCh. 5 - Prob. 27RQCh. 5 - Potassium permanganate is often used for redox...Ch. 5 - Assign oxidation numbers to the atoms in the...Ch. 5 - 5.30 Assign oxidation numbers to the atoms in the...Ch. 5 - Assign oxidation numbers to each atom in the...Ch. 5 - Assign oxidation numbers to the elements...Ch. 5 - 5.33 Assign oxidation numbers to the elements in...Ch. 5 - 5.34 Assign oxidation numbers to the elements in...Ch. 5 - Titanium burns in pure nitrogen to form TiN. What...Ch. 5 - 5.36 Zirconia, which is , is used to make ceramic...Ch. 5 - 5.37 Ozone, , is an allotrope of oxygen and is one...Ch. 5 - 5.38 The other major air pollutant is . What are...Ch. 5 - For the following reactions, identify the...Ch. 5 - 5.40 For the following reactions, identify the...Ch. 5 - When chlorine is added to drinking water to kill...Ch. 5 - One pollutant in smog is nitrogen dioxide. NO2....Ch. 5 - Balance the following equations for reactions...Ch. 5 - 5.44 Balance the following equations for reactions...Ch. 5 - Prob. 45RQCh. 5 - 5.46 Balance the following equations for reactions...Ch. 5 - 5.47 Balance the equations for the following...Ch. 5 - Balance the equations for the following reactions...Ch. 5 - Prob. 49RQCh. 5 - 5.50 Hydroiodic acid reduces chlorine to...Ch. 5 - Prob. 51RQCh. 5 - Corn is grown for human consumption, feeding...Ch. 5 - Prob. 53RQCh. 5 - Laundry bleach such as Clorox is a dilute solution...Ch. 5 - Prob. 55RQCh. 5 - Chlorine is a good bleaching agent because it is...Ch. 5 - Write balanced molecular, ionic, and net ionic...Ch. 5 - 5.58 Write balanced molecular, ionic, and net...Ch. 5 - Prob. 59RQCh. 5 - Prob. 60RQCh. 5 - Prob. 61RQCh. 5 - Prob. 62RQCh. 5 - Prob. 63RQCh. 5 - Hot, concentrated sulfuric acid is a fairly strong...Ch. 5 - Use Table 5.3 to predict the outcome of the...Ch. 5 - 5.66 Use Table 5.3 to predict the outcome of the...Ch. 5 - The following reactions occur spontaneously....Ch. 5 - The following reactions occur spontaneously....Ch. 5 - Prob. 69RQCh. 5 - When chromium metal is dipped into a solution of...Ch. 5 - Write a balanced equation for the reaction...Ch. 5 - Write a balanced equation for the reaction...Ch. 5 - Write the balanced chemical equation for the...Ch. 5 - Write the balanced chemical equation for the...Ch. 5 - 5.75 Write balanced chemical equations for the...Ch. 5 - Write balanced chemical equations for the complete...Ch. 5 - 5.77 Write balanced equations for the combustion...Ch. 5 - 5.78 Write balanced equations for the combustion...Ch. 5 - 5.79 The metabolism of carbohydrates such as...Ch. 5 - Methanol, CH3OH, has been suggested as an...Ch. 5 - Write the balanced equation for the combustion of...Ch. 5 - 5.82 Thiophene, , is an impurity in crude oil and...Ch. 5 - Write chemical equations for the reaction of...Ch. 5 - Prob. 84RQCh. 5 - Prob. 85RQCh. 5 - Prob. 86RQCh. 5 - In an acidic solution, permanganate ion reacts...Ch. 5 - 5.88 In an acidic solution, bisulfite ion reacts...Ch. 5 - Prob. 89RQCh. 5 - Potable water (drinking water) should not have...Ch. 5 - 5.91 Sulfites are used worldwide in the wine...Ch. 5 - Methylbromide, CH3Br, is used in agriculture to...Ch. 5 - A sample of a copper ore with a mass of 0.4225 g...Ch. 5 - 5.94 A 1.362 g sample of an iron ore that...Ch. 5 - 5.95 Hydrogen peroxide, , solution can be...Ch. 5 - 5.96 Sodium nitrite, , is used as a preservative...Ch. 5 - Prob. 97RQCh. 5 - Prob. 98RQCh. 5 - Both calcium chloride and sodium chloride are used...Ch. 5 - 5.100 One way to analyze a sample for nitrite ion...Ch. 5 - Use oxidation numbers to show that the...Ch. 5 - Prob. 102RQCh. 5 - Prob. 103RQCh. 5 - Prob. 104RQCh. 5 - Balance the following equations using the...Ch. 5 - Prob. 106RQCh. 5 - What is the average oxidation number of carbon in...Ch. 5 - Prob. 108RQCh. 5 - In Problem 5-108, were all of the experiments...Ch. 5 - Prob. 110RQCh. 5 - 5.111 In each of the following pairs, choose the...Ch. 5 - Use Table 5.3 to predict whether the following...Ch. 5 - Sucrose, C12H22O11, is ordinary table sugar. Write...Ch. 5 - Balance the following equations by the...Ch. 5 - 5.115 Lead(IV) oxide reacts with hydrochloric acid...Ch. 5 - Prob. 116RQCh. 5 - A copper bar with a mass of 12.340 g is dipped...Ch. 5 - Prob. 118RQCh. 5 - 5.119 As described in the Chemistry Outside the...Ch. 5 - Titanium(IV) can be reduced to titanium(III) by...Ch. 5 - A researcher planned to use chlorine gas in an...Ch. 5 - 5.122 A sample of a tin ore weighing 0.3000 g was...Ch. 5 - Biodiesel is formed from the reaction of oils with...Ch. 5 - *5.124 In June 2002, the Department of Health and...Ch. 5 - An organic compound contains carbon, hydrogen, and...Ch. 5 - *5.126 A mixture is made by combining with ....Ch. 5 - A solution containing 0.1244 g of K2C2O4 was...Ch. 5 - It was found that a 20.0 mL portion of a solution...Ch. 5 - A 15.00 mL sample of a solution containing oxalic...Ch. 5 - A solution with a volume of 500.0 mL contained a...Ch. 5 - Prob. 131RQCh. 5 - We described the ion-electron method for balancing...Ch. 5 - Assuming that a chemical reaction with DNA could...Ch. 5 - Would you expect atomic oxygen and chlorine to be...Ch. 5 - Prob. 135RQCh. 5 - Prob. 136RQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give your own example of how the structure of a part of the body is related to its function.
Principles of Anatomy and Physiology
Which one of the following is not a fuel produced by microorganisms? a. algal oil b. ethanol c. hydrogen d. met...
Microbiology: An Introduction
2. How many significant figures does each of the following numbers have?
a. 290 b. 2.90 × 104 c. 0.0029 d. 2.9...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Use the following graph to answer questions 3 and 4. 3. Which of the lines best depicts the log phase of a ther...
Microbiology: An Introduction
A simple magnifier of focal length f is placed near the eye of someone whose near point Pn is 25 cm. An object ...
Fundamentals of Physics Extended
Why are BSL-4 suits pressurized? Why not just wear tough regular suits?
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward
- € + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward
- 03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forward
- Calculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forwardAlcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forwardDraw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Acid-Base Titration | Acids, Bases & Alkalis | Chemistry | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=yFqx6_Y6c2M;License: Standard YouTube License, CC-BY