
Concept explainers
Without referring to Figure 5.1, write Lewis dot symbols for the following atoms/ions: (a) Be, (b) K, (c) Ca, (d) Ga, (e) O, (f) Li+, (g) Cl–, (h) S2−, (i) Sr2+, (j) N3−.
(a)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
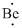
Explanation of Solution
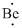
The electronic configuration of Beryllium is
(b)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
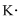
Explanation of Solution
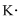
The electronic configuration of Potassium is
(c)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
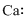
Explanation of Solution
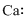
The electronic configuration of Calcium is
(d)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
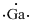
Explanation of Solution
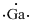
The electronic configuration of Gallium is
(e)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
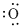
Explanation of Solution
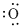
The electronic configuration of oxygen is
Lewis dot symbol denotes the valence electron of an atom. The valence electron of
(f)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
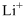
Explanation of Solution
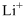
The electronic configuration of Lithium is
(g)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
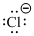
Explanation of Solution
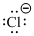
The electronic configuration of Chlorine is
(h)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
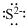
Explanation of Solution
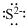
The electronic configuration of Sulphur is
(i)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
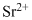
Explanation of Solution
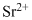
The electronic configuration of Strontium is
(j)
Interpretation:
Lewis dot symbol for the given atoms/ions has to be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
Answer to Problem 5.6QP
Lewis dot representation for
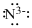
Explanation of Solution
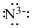
The electronic configuration of Nitrogen is
Want to see more full solutions like this?
Chapter 5 Solutions
GEN COMBO CHEMISTRY: ATOMS FIRST; ALEKS 360 2S ACCESS CARD CHEMISTRY:ATOMS FIRST
- Indicate the variation in conductivity with concentration in solutions of strong electrolytes and weak electrolytes.arrow_forwardThe molar conductivity of a very dilute solution of NaCl has been determined. If it is diluted to one-fourth of the initial concentration, qualitatively explain how the molar conductivity of the new solution will compare with the first.arrow_forwardWhat does the phrase mean, if instead of 1 Faraday of electricity, Q coulombs (Q/F Faradays) pass through?arrow_forward
- What characteristics should an interface that forms an electrode have?arrow_forwardFor a weak acid AcH, calculate the dissociated fraction (alpha), if its concentration is 1.540 mol L-1 and the concentration [H+] is 5.01x10-4 mol L-1.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forward
- If the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardIf the molar conductivity at infinite dilution of HAC is A0 = 390.5 S cm² mol¹. Calculate the Arrhenius conductivity of a 9.3% by weight solution of HAc with a pH of 3.3. Data: molecular weight of HAC is 60.05 g/mol and the density of the solution is 1 g/cm³.arrow_forwardDetermine the distance between the metal and the OHP layer using the Helm- holtz model when the electrode's differential capacitance is 145 μF cm². DATA: dielectric constant of the medium for the interfacial zone &r= lectric constant of the vacuum &0 = 8.85-10-12 F m-1 = 50, die-arrow_forward
- Describe a sequence of photophysical processes that can be followed by radiation adsorbed by a molecule in the ground state to give rise to phosphorescent emission.arrow_forwardState two similarities between fluorescence and phosphorescence.arrow_forwardState three photophysical processes that can be related to the effects of incident radiation on a molecule in its ground state. Consider that radiation can give rise to fluorescent emission, but not phosphorescent emission.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





