
Concept explainers
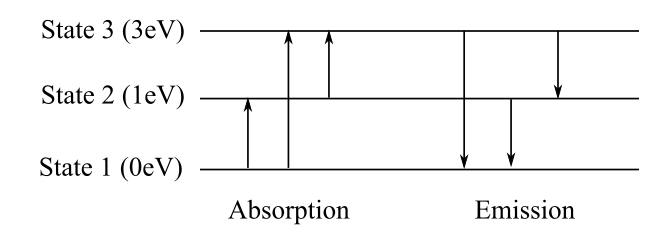
The diagram showing all possible transitions between the levels of an imaginary atom, having energy
Answer to Problem 41Q
Solution:
The diagram representing all possible transitions is shown below. The wavelengths for the energy differences
Explanation of Solution
Given data:
The energy values of the given atomic levels are
Formula used:
Planck’s quantum theory relates the energy difference between the transition levels to the frequency of the released
Or,
Here,
Explanation:
The given atom has only three energy levels. Let, state 1 represents the level with energy
Write the expression relating the energy and wavelength of the photon:
Here,
Estimate the energy difference of the levels.
The difference between first two energy states is,
Similarly,
And,
Rewrite the above expression for energy difference for wavelength.
Substitute
Substitute
All the possible transitions are shown in the following diagram.

The absorption or emission transition of visible lights are between the states 2 and 3 and 1 and 3 as the wavelength for these transitions lie in the visible region.
Conclusion:
The energy level diagram is shown above. The values of energy of the photons are
Want to see more full solutions like this?
Chapter 5 Solutions
EBK LOOSE-LEAF VERSION OF UNIVERSE
- Can someone help me with this physics 2 problem thank you.arrow_forwardTwo concrete spans of a 234 m long bridge are placed end to end so that no room is allowed for expansion (Figure a). Each span therefore has a length of L0 = 117 m. If the temperature increases by 17.8 °C, what is the height y to which the spans rise when they buckle (Figure b)? (The coefficient of linear expansion of concrete is 1.20⋅10−51.20⋅10-5 °C−1.)arrow_forwardMonkey D. Luffy, from One Piece can inflate himself like a balloon to a size of 6.98 m3 by inhaling 1.74⋅10^26 molecules of air. If the air is at 20.9 ˚C, the pressure inside Luffy is 101277.062 Pa. kB=1.38⋅10^−23 J/K. The total internal energy of the gas inside Luffy is 1065333.93 J. How fast, on average, is the air molecules inside Luffy traveling at? The average mass of an air molecule (considering the various gasses involved) is 4.51 x 10^-26 kg.arrow_forward
- The Dungeons & Dragons spell “Stinking Cloud” fills a 949 m^3 volume of air with a cloud of gas. The pressure of the gas is the same as the air, 101,325 Pa, and is at 29.2°C. There are 2.304x10^28 molecules of gas. What is the total internal energy of the gas?arrow_forwardThe Fiero, which is 4.70 m long, starts at 10.0˚C while in the upper atmosphere but when it goes into space the temperature would be about -270.3˚C. How much should the steel siding of the Fiero shrink due to this temperature change? The coefficient of thermal linear expansion for steel is 11.0⋅10−6⋅10^-6 C-1arrow_forwardQuestion 3 of 17 L X L L T 0.5/ In the figure above, three uniform thin rods, each of length L, form an inverted U. The vertical rods each have a mass m; the horizontal rod has a mass 3m. NOTE: Express your answer in terms of the variables given. (a) What is the x coordinate of the system's center of mass? xcom L 2 (b) What is the y coordinate of the system's center of mass? Ycom 45 L X Q Search MD bp Narrow_forward
- Sketch the harmonic on graphing paper.arrow_forwardExercise 1: (a) Using the explicit formulae derived in the lectures for the (2j+1) × (2j + 1) repre- sentation matrices Dm'm, (J/h), derive the 3 × 3 matrices corresponding to the case j = 1. (b) Verify that they satisfy the so(3) Lie algebra commutation relation: [D(Î₁/ħ), D(Î₂/h)]m'm₁ = iƊm'm² (Ĵ3/h). (c) Prove the identity 3 Dm'm,(β) = Σ (D(Ρ)D(Ρ))m'¡m; · i=1arrow_forwardSketch the harmonic.arrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College





