
Interpretation: The configuration and chirality should be identified for the given molecule by using its structure.
Concept Introduction:
Chiral carbon: Chiral atom is the one which is bonded to four different molecules or groups.
Configuration of a molecule: The configurations of a molecule arise due to the spatial arrangement of atoms. The configuration can be assigned by following CIP rules as follows.
Rules
Find the chiral carbon atom in a molecule.
Assign numbering to the groups bonded to the chiral carbon based on the molecular weight and electronegativity.
If the sequence of the numbering follows clockwise direction the chiral atom is assigned as R configuration.
If the sequence of the numbering follows anticlockwise direction the chiral atom is assigned as S configuration
The R and S configuration of a molecule can be interchanged when a least prior group present in above plane.
To find: The chiral atoms present of the given molecule.
Answer to Problem 31PP
The R and S nomenclature for the given molecule are shown below.
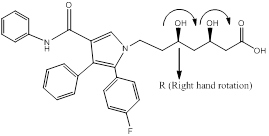
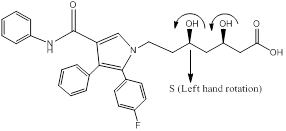
Explanation of Solution
- Draw the structure of the given molecule and find the chiral centers.
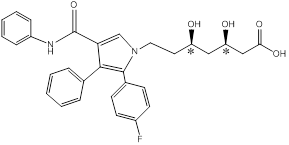
Chiral centers of the molecule are indicated by using *
The chiral center in the given molecule is marked using*. The * Carbons are chiral since all the carbons are
The presence of an asymmetric carbon center is one of several structural features that induce chirality in organic molecules.
- Assign absolute configuration R-S for the given molecules using CIP rules.
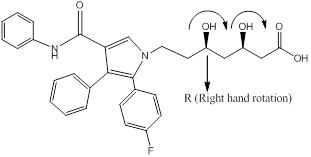
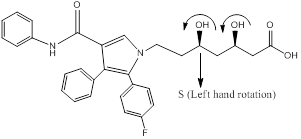
Chiral carbons are labeled as shown above molecules. Further the right and left hand nomenclature is used to name the enantiomers of chiral compounds. The stereocenter are indicated as R or S.
From the priority is assigned of the above molecules, according to the substitution of elements with higher
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY (LL) W/ACCESS
- Done 19:17 www-awu.aleks.com Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimited .III LTE סוי 27 28 = 29 30 31 32 = 33 34 35 Consider this structure. CH3CH2CH2 Part 1 of 3 3 CH2 CH2CH3 - C-CH2CH 3 H CH₂ Give the IUPAC name of this structure. 3-ethyl-3,4-dimethylheptane Part: 1/3 Part 2 of 3 Draw the skeletal structure. Skip Part < Check Click and drag to start drawing a structure. Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Хarrow_forward18:57 .III LTE www-awu.aleks.com Chapter 12 HW Question 31 of 39 (8 points) | Question Attem... Give the IUPAC name of each compound. Part 1 of 4 Part 2 of 4 Х Х Check Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. TOMS OF US vacy Center | Accessibilityarrow_forwardWhat is the missing reactant in this organic reaction? CH3-C-CH2-NH2 + R - CH3 O: 0 CH3-N-CH2-C-NH-CH2-C-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click anywhere to draw the first atom of your structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forward
- Done 18:17 • www-awu.aleks.com Chapter 12 HW Question 24 of 39 (4 points) | Question Attempt: 1 of Unlimited ▼ 20 ✓ 21 × 22 23 24 25 26 raw the structure corresponding to each IUPAC name. Part 1 of 2 .III LTE 22 27 28 סוי 29 29 3 A skeletal structure corresponding to the IUPAC name 3-ethyl-4-methylhexane. Part 2 of 2 Click and drag to start drawing a structure. A condensed structure corresponding to the IUPAC name 2,2,4- trimethylpentane. Click anywhere to draw the first atom of your structure. Check Save For Later Submit < Х ப: G © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility : Garrow_forwardDone 18:25 www-awu.aleks.com .III LTE Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimi... Oli 23 24 25 26 27 28 29 30 Consider this structure. CH2 CH2CH2 CH2CH2CH₂ C -C. -CH2CH3 H CH Part: 0 / 3 Part 1 of 3 Give the IUPAC name of this structure. Skip Part < Check ☑ Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility ....................arrow_forwardCalculate Ecell at 25.0 oC using the following line notation. Zn(s)|Zn+2(aq, 0.900 M)||Cu+2(aq, 0.000200 M)|Cu(s)arrow_forward
- Predict the product of this organic reaction: O OH + H + OH A P + H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click and drag to start drawing a structure. X G ☐ :arrow_forward0.0994 g of oxalic acid dihydrate is titrated with 10.2 mL of potassium permanganate. Calculate the potassium permanganate concentration. Group of answer choices 0.0433 M 0.135 M 0.0309 M 0.193 Marrow_forwardExperts...can any one help me solve these problems?arrow_forward
- According to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardWhich Group 1 metal reacts with O2(g) to form a metal peroxide (M2O2)? Group of answer choices Li K Rb Naarrow_forwardWhich of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





