
Concept explainers
Figure 5.7 On a hot, dry day, plants close their stomata to conserve Water. What impact will this have on photosynthesis?
To write:
Description of the impact of the closing of stomata on photosynthesis during hot dry condition.
Introduction:
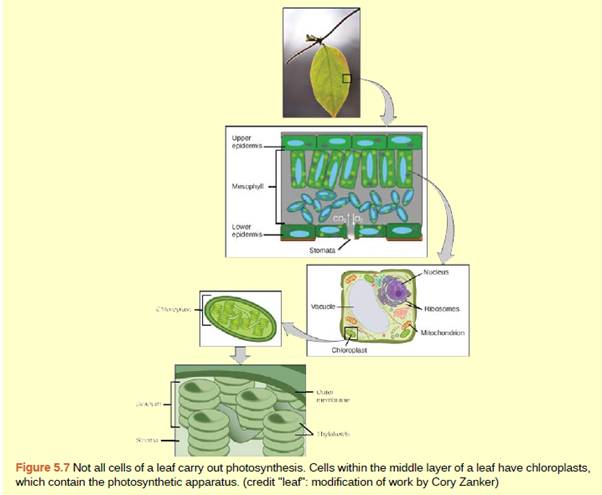
Stomata are the small opening that exists in the membranes of the plants through which exchange of gas and water takes place. Stomata play an important role in the intake and transpiration of the CO2 which is significant for the process of photosynthesis.
Explanation of Solution
During hot dry conditions, the stomata close its opening to conserve water. The humidity around the leaves is affected by the temperature of the air around it. If the temperature of the air is higher, the water from the leaves will diffuse more into the air. But it is noted that the leaf has a waxy cuticle blocking the water loss whereas carbon dioxide and oxygen exchange with the environment is required for photosynthesis.
There are specialized epidermal cells around the stomata known as guard cells. These guard cells swell up when there is enough water in the cells opening the stomata. But in case the water is less, these guard cells don't swell and hence the stomata remain closed so that the plant does not lose water. If the stomata are closed, then there is no photosynthesis and the plant will starve.
During the hot dry season, as there is not enough water in the cells, the guard cells do not swell keeping the stomata closed. As result, photosynthesis does not take place and eventually cause starvation of the plant.
Want to see more full solutions like this?
Chapter 5 Solutions
Concepts of Biology
Additional Science Textbook Solutions
Concepts of Genetics (12th Edition)
Biological Science (6th Edition)
Microbiology with Diseases by Body System (5th Edition)
Human Anatomy & Physiology (2nd Edition)
Organic Chemistry (8th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
- When setting up a PCR reaction to act as a negative control for the surface protein A gene... Which primers will you add to the reaction mix? mecA primers, spa primers, mecA primers and spa primers, no primers What will you add in place of template? sterile water, MRSA DNA, Patient DNA, S. aureus DNAarrow_forwardDraft a science fair project for a 11 year old based on the human body, specifically the liverarrow_forwardYou generate a transgenic mouse line with a lox-stop-lox sequence upstream of a dominant-negative Notch fused to GFP. Upon crossing this mouse with another mouse line expressing ectoderm-specific Cre, what would you expect for the phenotype of neuronal differentiation in the resulting embryos?arrow_forward
- Hair follicle formation is thought to result from a reaction-diffusion mechanism with Wnt and its antagonist Dkk1. How is Dkk1 regulated by Wnt? Describe specific cis-regulatory elements and the net effect on Dkk1 expression.arrow_forwardLimetown S1E4 Transcript: E n 2025SP-BIO-111-PSNT1: Natu X Natural Selection in insects X + newconnect.mheducation.com/student/todo CA NATURAL SELECTION NATURAL SELECTION IN INSECTS (HARDY-WEINBERG LAW) INTRODUCTION LABORATORY SIMULATION A Lab Data Is this the correct allele frequency? Is this the correct genotype frequency? Is this the correct phenotype frequency? Total 1000 Phenotype Frequency Typica Carbonaria Allele Frequency 9 P 635 823 968 1118 1435 Color Initial Frequency Light 0.25 Dark 0.75 Frequency Gs 0.02 Allele Initial Allele Frequency Gs Allele Frequency d 0.50 0 D 0.50 0 Genotype Frequency Moths Genotype Color Moths Released Initial Frequency Frequency G5 Number of Moths Gs NC - Xarrow_forwardWhich of the following is not a sequence-specific DNA binding protein? 1. the catabolite-activated protein 2. the trp repressor protein 3. the flowering locus C protein 4. the flowering locus D protein 5. GAL4 6. all of the above are sequence-specific DNA binding proteinsarrow_forward
- Which of the following is not a DNA binding protein? 1. the lac repressor protein 2. the catabolite activated protein 3. the trp repressor protein 4. the flowering locus C protein 5. the flowering locus D protein 6. GAL4 7. all of the above are DNA binding proteinsarrow_forwardWhat symbolic and cultural behaviors are evident in the archaeological record and associated with Neandertals and anatomically modern humans in Europe beginning around 35,000 yBP (during the Upper Paleolithic)?arrow_forwardDescribe three cranial and postcranial features of Neanderthals skeletons that are likely adaptation to the cold climates of Upper Pleistocene Europe and explain how they are adaptations to a cold climate.arrow_forward
- Biology Questionarrow_forward✓ Details Draw a protein that is embedded in a membrane (a transmembrane protein), label the lipid bilayer and the protein. Identify the areas of the lipid bilayer that are hydrophobic and hydrophilic. Draw a membrane with two transporters: a proton pump transporter that uses ATP to generate a proton gradient, and a second transporter that moves glucose by secondary active transport (cartoon-like is ok). It will be important to show protons moving in the correct direction, and that the transporter that is powered by secondary active transport is logically related to the proton pump.arrow_forwarddrawing chemical structure of ATP. please draw in and label whats asked. Thank you.arrow_forward
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
 Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning





