
Sustainable Energy, Si Edition
2nd Edition
ISBN: 9781337551670
Author: DUNLAP, Richard A.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 11P
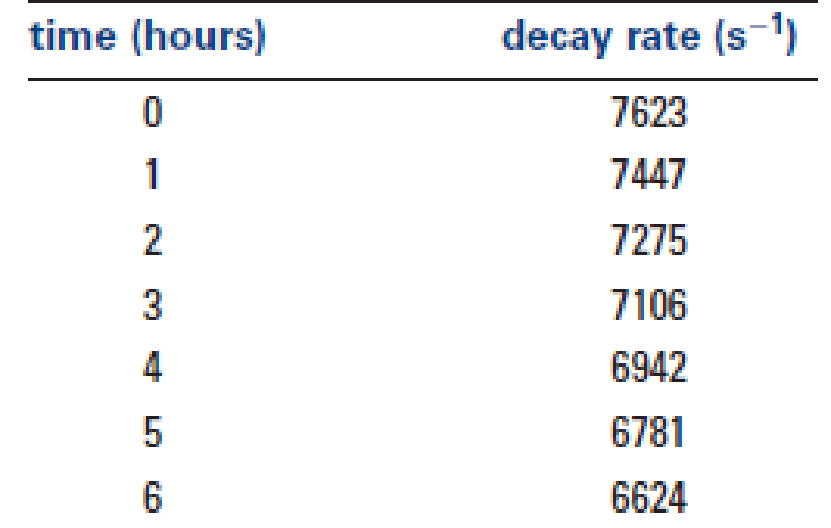
The measured decay rates of a radioactive material as a function of time are shown in the table below.
- (a) Calculate the half-life of the decay.
- (b) Show that a semilogarithmic plot of the decay rate as a function of time is linear.
- (c) Derive an analytical form of the linear relationship for part (b).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Figure 1 shows the plan view of an Exhibition Hall with dimensions L1 x L2. The structure is to be constructed in a coastal region which will be exposed to mostly mild environmental conditions and should provide a 1.5-hour fire resistance. It is known that the underlying foundation soil contains sulphate and other organic compounds. The layout of the structure consists of portal frames which are spaced evenly along the L2 direction. The frames are to support a slab of thickness (t) that will cover the full area of the hall plan. The dead and live loads applied on the slab are G and Q (kPa), respectively.
A sample of Achilles saturated with water has a mass of 1710 g. After heating in an oven, a constant mass of 1815 g is obtained. The density of solid Achilles seeds is 2.78 g/cm3. We are asked to calculate:
a) The water content and void ratio
b) The porosity and specific gravity of the clayey soil
c) The wet density of the clayey soil, the corresponding dry density and dry density
8. A prestressed concrete beam is subjected to the
following stress distributions:
Pi is the initial prestressing force, Pe is the effective
prestressing force, M, is the bending moment due to self-
weight, Ma and M, are the dead load and live load bending
moment, respectively.
The concrete has the following properties: fr = 6000 psi
and fri = 4200 psi
+250 -85 -2500
+550
Pe+ Mo+Ma+Mi
P alone
P₁+ Mo
-2450 -3500
Stress at midspan
+210
+250
P, alone
Pe alone
-2500 -3500
Stress at ends
Using Table 22.1, evaluate whether the stresses at the center of the span and the end of the span comply with the
permissible stress limits. The beam is classified as U-class.
Provide justifications for each condition listed in the table.
Note: Calculated stresses are to be taken from the above diagram, and permissible stresses are to be calculated
using Table 22.1.
Compressive stresses
immediately after transfer
Tensile stresses
immediately after transfer
Compressive stresses
under sustained and total…
Chapter 5 Solutions
Sustainable Energy, Si Edition
Ch. 5 - Prob. 1PCh. 5 - Prob. 2PCh. 5 - A sample of 137Cs (half-life = 30.1 years) decays...Ch. 5 - Prob. 4PCh. 5 - What are the decay products of the decay of the...Ch. 5 - Prob. 6PCh. 5 - Nuclide A has a half-life of 4 days, and nuclide B...Ch. 5 - 64Cu can decay by both decay and + decay. Draw an...Ch. 5 - At time t = 0 a sample contains 3.57 108 nuclei...Ch. 5 - Prob. 10P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Similar questions
- 10. A short column is subjected to an eccentric loading. The axial load P = 1000 kips and the eccentricity e = 12 in. The material strengths are fy = 60 ksi and f = 6000 psi. The Young's modulus of steel is 29000 ksi. (a) Fill in the blanks in the interaction diagram shown below. (2pts each, 10pt total) Po Pn (1) failure range H 3" 30" Ast 6 No. 10 bars = P 22" I e H 3" (4) e = e small Load path for given e Radial lines show constant (2) eb (3) e large failure range Mn (5) e= Mo (b) Compute the balanced failure point, i.e., P and Mb.arrow_forwardNo chatgpt plsarrow_forward11. The prestressed T beam shown below is pretensioned using low relaxation stress-relieved Grade 270 strands. The steel area Aps = 2.5 in². The tensile strength is fpu = 270 ksi, and the concrete compressive strength is fr = 6000 psi. (a) Calculate the nominal moment strength Mn with hr = 6 in. 22" 15" T hf (b) Since this beam is a T-beam, the nominal moment strength M₁ increases with a thicker hf. However, M, stops increasing if he reaches a value. Determine the minimum thickness hy that can achieve the maximum nominal moment strength Mr. Also, calculate the corresponding maximum nominal moment strength Mn with the computed hf.arrow_forward
- 10. A short column is subjected to an eccentric loading. The axial load P = 1000 kips and the eccentricity e = 12 in. The material strengths are fy = 60 ksi and f = 6000 psi. The Young's modulus of steel is 29000 ksi. (a) Fill in the blanks in the interaction diagram shown below. 30" Ast 6 No. 10 bars = Pn (1) Po (4) e = e small Load path for given e failure range Radial lines show constant (2) eb (3) e large failure range Mn (5) e= Mo (b) Compute the balanced failure point, i.e., P and Mb. H 3" P 22" I e H 3"arrow_forward10. A short column is subjected to an eccentric loading. The axial load P = 1000 kips and the eccentricity e = 12 in. The material strengths are fy = 60 ksi and f = 6000 psi. The Young's modulus of steel is 29000 ksi. (a) Fill in the blanks in the interaction diagram shown below. 30" Ast 6 No. 10 bars = Pn (1) Po (4) e = e small Load path for given e failure range Radial lines show constant (2) eb (3) e large failure range Mn (5) e= Mo (b) Compute the balanced failure point, i.e., P and Mb. H 3" P 22" I e H 3"arrow_forward7. Match the given strand profiles with the corresponding loading conditions for a prestressed concrete (PSC) beam. Strand profile (b) (d) (c) (a) Ꮎ Load on a beamarrow_forward
- 4. For serviceability considerations, the effective moment of inertia (Ie) is calculated using the following formula: le 1 - 1cr ((2/3) Mcr) Ma 2 - وا ≥ Note that the upper bound was previously set as Iut in the earlier ACI equation. (a) Arrange the following moment of inertia values in ascending order (from smallest to largest): le, Ier, Ig and lut (b) Mer is the cracking moment. Choose the cross-section that should be used to compute Mcr. NA. h 5. Identify and circle the figure that represents the scenario in which the torsional effect is permitted to be reduced according to the ACI code provisions. (3 pts) mt mi B (b)arrow_forwardI will rate, thanksarrow_forward. 9. A reinforced concrete beam is subjected to V/ = 40 kips and Tu/ = 12 ft kips at the critical section. Given conditions: ⚫ Longitudinal reinforcements use No. 8 grade 60 steel with an effective depth d = 20 in. For shear capacity, V = 18 kips and V₂ = 22 kips • For transverse reinforcements, use No. 3 bars with grade 60. • The effective torsional area of A. = 150 in². • Crack angle = 45° ⚫ The minimum stirrup spacing is Smin = 4" and the maximum stirrup spacing is Smax = Find the required stirrup spacing at the critical section. 8".arrow_forward
- 3. The beam shown on the right uses three No. 8 bars made of Grade 60 steel as longitudinal reinforcement. The allowable maximum center-to-center spacing of the longitudinal rebars has been determined to be 10 inches. Now assume that Grade 80 steel will be used instead. Determine whether the beam satisfies the rebar spacing requirements according to the ACI Code. Additional assumptions: • Estimate fs = fy • 20" Clear cover: ? 12" Clear side cover: 1.5" The clear cover depth cc and the clear side cover remain unchanged, regardless of the change in material.arrow_forward6. For the slender columns shown below: a) Determine the effective buckling length factor (k) for each column. b) Circle the column with the largest buckling capacity, assuming all columns have the same length (f) and the same flexural rigidity (E+I) k = (a) (b) (c) (d)arrow_forward5. Identify and circle the figure that represents the scenario in which the torsional effect is permitted to be reduced according to the ACI code provisions. mi (a) V7+ B (b)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning
Materials Science And Engineering PropertiesCivil EngineeringISBN:9781111988609Author:Charles GilmorePublisher:Cengage Learning Engineering Fundamentals: An Introduction to Engi...Civil EngineeringISBN:9781305084766Author:Saeed MoaveniPublisher:Cengage Learning
Engineering Fundamentals: An Introduction to Engi...Civil EngineeringISBN:9781305084766Author:Saeed MoaveniPublisher:Cengage Learning


Materials Science And Engineering Properties
Civil Engineering
ISBN:9781111988609
Author:Charles Gilmore
Publisher:Cengage Learning

Engineering Fundamentals: An Introduction to Engi...
Civil Engineering
ISBN:9781305084766
Author:Saeed Moaveni
Publisher:Cengage Learning
The Science Inside Concrete Binders; Author: Dow;https://www.youtube.com/watch?v=GZq3Cyn2mVE;License: Standard Youtube License