
ORGANIC CHEMISTRY-EBOOK>I<
9th Edition
ISBN: 9781305084414
Author: McMurry
Publisher: INTER CENG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4.6, Problem 14P
Interpretation Introduction
Interpretation:
The coloured–green, red or blue colour bond is to be identified in cyclohexane in the starting and the flipped structure.
Concept introduction:
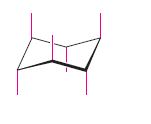
Axial bond in cyclohexane
Equatorial bond in cyclohexane
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
At 300 K, in the decomposition reaction
of a reactant R into products, several
measurements of the concentration of R
over time have been made (see table).
Without using graphs, calculate the
order of the reaction.
t/s
[R]/(mol L-1)
0
0,5
171
0,16
720
0,05
1400
0,027
Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.
What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.
Chapter 4 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
Ch. 4.1 - Give IUPAC names for the following cycloalkanes:Ch. 4.1 - Draw structures corresponding to the following...Ch. 4.1 - Name the following cycloalkane:Ch. 4.2 - Prob. 4PCh. 4.2 - Draw the structures of the following molecules:...Ch. 4.2 - Prostaglandin F2α, a hormone that causes uterine...Ch. 4.2 - Name the following substances, including the cis-...Ch. 4.3 - Each H↔H eclipsing interaction in ethane costs...Ch. 4.3 - cis-1, 2-Dimethylcyclopropane has more strain than...Ch. 4.4 - Prob. 10P
Ch. 4.4 - Two conformations of cis-l, 3-dimethylcyclobutane...Ch. 4.6 - Draw two different chair conformations of...Ch. 4.6 - Draw two differant chair conformations of trans-1,...Ch. 4.6 - Prob. 14PCh. 4.7 - What is the energy difference between the axial...Ch. 4.7 - Prob. 16PCh. 4.7 - Look at Figure 4-12 on page 105, and estimate the...Ch. 4.8 - Draw the more stable chair conformation of the...Ch. 4.8 - Identify each substituent in the following...Ch. 4.9 - Which isomer is more stable, cis-decalin or...Ch. 4.9 - Look at the following structure of the female...Ch. 4.SE - Prob. 22VCCh. 4.SE - Name the following compound, identify each...Ch. 4.SE - A trisubstituted cyclohexane with three...Ch. 4.SE - The following cyclohexane derivative has three...Ch. 4.SE - Prob. 26VCCh. 4.SE - Draw the five cycloalkanes with the formula C5H10.Ch. 4.SE - Draw two constitutional isomers of cis-1,...Ch. 4.SE - Prob. 29APCh. 4.SE - Tell whether the following pairs of compounds are...Ch. 4.SE - Prob. 31APCh. 4.SE - Prob. 32APCh. 4.SE - Draw 1, 3, 5-trimethylcyclohexane using a hexagon...Ch. 4.SE - Hydrocortisone, a naturally occurring hormone...Ch. 4.SE - A 1, 2-cis disubstituted cyclohexane, such as...Ch. 4.SE - A 1, 2-trans disubstituted cyclohexane must have...Ch. 4.SE - Prob. 37APCh. 4.SE - Which is more stable, a 1, 4-trans disubstituted...Ch. 4.SE - cis-1, 2-Dimethylcyclobutane is less stable than...Ch. 4.SE - From the data in Figure 4-12 and Table 4-1,...Ch. 4.SE - Prob. 41APCh. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Galactose, a sugar related to glucose, contains a...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - The diaxial conformation of cis-1,...Ch. 4.SE - Approximately how much steric strain does the...Ch. 4.SE - In light of your answer to Problem 4-43, draw the...Ch. 4.SE - Prob. 50APCh. 4.SE - Prob. 51APCh. 4.SE - Using molecular models as well as structural...Ch. 4.SE - trans-Decalin is more stable than its cis isomer,...Ch. 4.SE - As mentioned in Problem 3-53, the statin drugs,...Ch. 4.SE - myo-Inositol, one of the isomers of...Ch. 4.SE - How many cis–trans stereoisomers of myo-inositol...Ch. 4.SE - The German chemist J. Bredt proposed in 1935 that...Ch. 4.SE - Tell whether each of the following substituents on...Ch. 4.SE - Prob. 59APCh. 4.SE - Prob. 60APCh. 4.SE - Ketones react with alcohols to yield products...Ch. 4.SE - Alcohols undergo an oxidation reaction to yield...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat would happen if you added the HCI to the Grignard reagent before adding benzophenone? Draw a reaction mechanism to support your answer.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Calculate the order of the reaction. t/s [R]/ (mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Write the correct IUPAC names of the molecules in the picturearrow_forwardHow many grams of solid NaCN have to be added to 1.5L of water to dissolve 0.18 mol of Fe(OH)3 in the form Fe(CN)63 - ? ( For simplicity, ignore the reaction of CN - ion with water) Ksp for Fe(OH)3 is 2.8E -39, and Kform for Fe(CN)63 - is 1.0E31arrow_forwardDraw the most stable chair conformation of 1-ethyl-1-methylcyclohexane, clearly showing the axial and equatorial substituents. [4] Draw structures corresponding to the following IUPAC name for each of the following compounds; [5] i) 4-Isopropyl-2,4,5-trimethylheptane ii) trans-1-tert-butyl-4-ethylcyclohexane iii) Cyclobutylcycloheptane iv) cis-1,4-di-isopropylcyclohexane (chair conformation) v) 3-Ethyl-5-isobutylnonanearrow_forward
- Draw and name molecules that meet the following descriptions; [4] a) An organic molecule containing 2 sp2 hybridised carbon and 1 sp-hybridised carbon atom. b) A cycloalkene, C7H12, with a tetrasubstituted double bond. Also answer question 2 from the imagearrow_forwardH 14. Draw the line angle form of the following molecule make sure you use the proper notation to indicate spatial positioning of atoms. F F H 15. Convert the following condensed form to line angle form: (CH3)3CCH2COCH2CON(CH2CH3)2arrow_forwardIn a reaction between two reactants A and B, the half-life is the same for both only if(A) the stoichiometry A:B is 1:1.(B) the stoichiometry A:B is 1:2 or 2:1.arrow_forward
- In a reaction between two reactants A and B, the half-life is the same for both.(1) Only if the stoichiometry A:B is 1:1.(2) If the initial quantities of A and B are in their stoichiometric ratios.arrow_forwardThere are 48 pairs of students in the following table. Each pair has quantitatively determined the mass of taurine in a 250 mL can of the popular energy drink marketed as “Munster” using High Performance Liquid Chromatography (HPLC). The class results are presented below: QUESTION: Calculate the measurement of uncertainty and provide the data in a spreadsheet table. Mass of Taurine (mg) Mass of Taurine (mg) (Table continued) 152.01 152.23 151.87 151.45 154.11 152.64 152.98 153.24 152.88 151.45 153.49 152.48 150.68 152.33 151.52 153.63 152.48 151.68 153.17 153.40 153.77 153.67 152.34 153.16 152.57 153.02 152.86 151.50 151.23 152.57 152.72 151.54 146.47 152.38 152.44 152.54 152.53 152.54 151.32 152.87 151.24 153.26 152.02 152.90 152.87 151.49 152.46 152.58arrow_forward1. Predict the organic product(s) of the following reactions. Assume excess of reagents unless otherwise noted. a) &l BH3 •THF b) 1) NaOH 2) H3O+ solve d) ala 1) EtMgBr 2) H3O+ e) H2N سكر CuLi NH2 1) SOCI2 2) EtMgBr 3) H3O+ NC H3O+ Δarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY