
EBK THERMODYNAMICS: AN ENGINEERING APPR
9th Edition
ISBN: 8220106796979
Author: CENGEL
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.5, Problem 72P
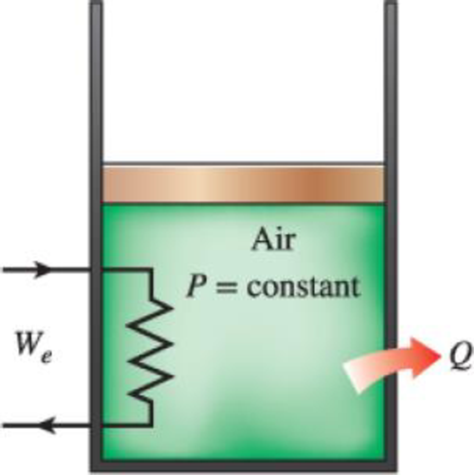
A mass of 15 kg of air in a piston–cylinder device is heated from 25 to 95°C by passing current through a resistance heater inside the cylinder. The pressure inside the cylinder is held constant at 300 kPa during the process, and a heat loss of 60 kJ occurs. Determine the electric energy supplied, in kWh.
FIGURE P4–72
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
can you explain how in a coordinate frame transformation: v = {v_n}^T {n-hat} and then it was found that {n-hat} = [C]^T {b-hat} so v_n = {v_n}^T [C]^T {b-hat}, how does that equation go from that to this --> v_n = [C]^T v_b
6) If (k = 0,7 cm) find Imax for figure below.
225mm
100mm
ثلاثاء.
100mm
150mm
75mm
Ans: Tmax=45:27 N/cm
F-400 N
The man has a weight W and stands halfway along the beam. The beam is not smooth, but the planes at A and B are smooth (and plane A is horizontal). Determine the magnitude of the tension in the cord in terms of W and θ.
Chapter 4 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
Ch. 4.5 - Is the boundary work associated with...Ch. 4.5 - On a P-V diagram, what does the area under the...Ch. 4.5 - An ideal gas at a given state expands to a fixed...Ch. 4.5 - Calculate the total work, in kJ, for process 13...Ch. 4.5 - Calculate the total work, in Btu, produced by the...Ch. 4.5 - Nitrogen at an initial state of 300 K, 150 kPa,...Ch. 4.5 - The volume of 1 kg of helium in a pistoncylinder...Ch. 4.5 - A pistoncylinder device with a set of stops...Ch. 4.5 - A mass of 5 kg of saturated water vapor at 150 kPa...Ch. 4.5 - A frictionless pistoncylinder device contains 16...
Ch. 4.5 - 1 m3 of saturated liquid water at 200C is expanded...Ch. 4.5 - Argon is compressed in a polytropic process with n...Ch. 4.5 - A gas is compressed from an initial volume of 0.42...Ch. 4.5 - A mass of 1.5 kg of air at 120 kPa and 24C is...Ch. 4.5 - During some actual expansion and compression...Ch. 4.5 - A frictionless pistoncylinder device contains 5 kg...Ch. 4.5 - During an expansion process, the pressure of a gas...Ch. 4.5 - A pistoncylinder device initially contains 0.4 kg...Ch. 4.5 - A pistoncylinder device contains 0.15 kg of air...Ch. 4.5 - Determine the boundary work done by a gas during...Ch. 4.5 - 1 kg of water that is initially at 90C with a...Ch. 4.5 - An ideal gas undergoes two processes in a...Ch. 4.5 - A pistoncylinder device contains 50 kg of water at...Ch. 4.5 - Prob. 26PCh. 4.5 - A closed system like that shown in Fig. P427E is...Ch. 4.5 - A rigid container equipped with a stirring device...Ch. 4.5 - Complete each line of the following table on the...Ch. 4.5 - A substance is contained in a well-insulated rigid...Ch. 4.5 - A 0.5-m3rigid tank contains refrigerant-134a...Ch. 4.5 - A 20-ft3 rigid tank initially contains saturated...Ch. 4.5 - A rigid 10-L vessel initially contains a mixture...Ch. 4.5 - A rigid 1-ft3 vessel contains R-134a originally at...Ch. 4.5 - A pistoncylinder device contains 5 kg of...Ch. 4.5 - A pistoncylinder device contains 0.5 lbm of water...Ch. 4.5 - 2 kg of saturated liquid water at 150C is heated...Ch. 4.5 - An insulated pistoncylinder device contains 5 L of...Ch. 4.5 - A 40-L electrical radiator containing heating oil...Ch. 4.5 - Steam at 75 kPa and 8 percent quality is contained...Ch. 4.5 - A pistoncylinder device initially contains 0.6 m3...Ch. 4.5 - An insulated tank is divided into two parts by a...Ch. 4.5 - Two tanks (Tank A and Tank B) are separated by a...Ch. 4.5 - Is the energy required to heat air from 295 to 305...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - A fixed mass of an ideal gas is heated from 50 to...Ch. 4.5 - Is the relation u = mcv,avgT restricted to...Ch. 4.5 - Is the relation h = mcp,avgT restricted to...Ch. 4.5 - What is the change in the internal energy, in...Ch. 4.5 - Neon is compressed from 100 kPa and 20C to 500 kPa...Ch. 4.5 - What is the change in the enthalpy, in kJ/kg, of...Ch. 4.5 - A mass of 10 g of nitrogen is contained in the...Ch. 4.5 - Determine the internal energy change u of...Ch. 4.5 - Determine the enthalpy change h of oxygen, in...Ch. 4.5 - Is it possible to compress an ideal gas...Ch. 4.5 - Nitrogen in a rigid vessel is cooled by rejecting...Ch. 4.5 - Nitrogen at 100 psia and 300F in a rigid container...Ch. 4.5 - A pistoncylinder device containing carbon-dioxide...Ch. 4.5 - A 3-m3 rigid tank contains hydrogen at 250 kPa and...Ch. 4.5 - 1 kg of oxygen is heated from 20 to 120C....Ch. 4.5 - A 10-ft3 tank contains oxygen initially at 14.7...Ch. 4.5 - A 4-m 5-m 7-m room is heated by the radiator of...Ch. 4.5 - An insulated rigid tank is divided into two equal...Ch. 4.5 - An ideal gas contained in a pistoncylinder device...Ch. 4.5 - A 4-m 5-m 6-m room is to be heated by a...Ch. 4.5 - An insulated pistoncylinder device initially...Ch. 4.5 - Argon is compressed in a polytropic process with n...Ch. 4.5 - An insulated pistoncylinder device contains 100 L...Ch. 4.5 - Air is contained in a variable-load pistoncylinder...Ch. 4.5 - A mass of 15 kg of air in a pistoncylinder device...Ch. 4.5 - Prob. 73PCh. 4.5 - A pistoncylinder device contains 2.2 kg of...Ch. 4.5 - A pistoncylinder device contains 4 kg of argon at...Ch. 4.5 - A spring-loaded pistoncylinder device contains 5...Ch. 4.5 - Prob. 78PCh. 4.5 - Prob. 79PCh. 4.5 - A 1-kg block of iron is heated from 25 to 75C....Ch. 4.5 - The state of liquid water is changed from 50 psia...Ch. 4.5 - During a picnic on a hot summer day, all the cold...Ch. 4.5 - An ordinary egg can be approximated as a...Ch. 4.5 - Consider a 1000-W iron whose base plate is made of...Ch. 4.5 - Stainless steel ball bearings ( = 8085 kg/m3 and...Ch. 4.5 - In a production facility, 1.6-in-thick 2-ft 2-ft...Ch. 4.5 - Long cylindrical steel rods ( = 7833 kg/m3 and cp...Ch. 4.5 - An electronic device dissipating 25 W has a mass...Ch. 4.5 - Prob. 90PCh. 4.5 - Prob. 91PCh. 4.5 - Is the metabolizable energy content of a food the...Ch. 4.5 - Is the number of prospective occupants an...Ch. 4.5 - Prob. 94PCh. 4.5 - Prob. 95PCh. 4.5 - Prob. 96PCh. 4.5 - Consider two identical 80-kg men who are eating...Ch. 4.5 - A 68-kg woman is planning to bicycle for an hour....Ch. 4.5 - A 90-kg man gives in to temptation and eats an...Ch. 4.5 - A 60-kg man used to have an apple every day after...Ch. 4.5 - Consider a man who has 20 kg of body fat when he...Ch. 4.5 - Consider two identical 50-kg women, Candy and...Ch. 4.5 - Prob. 103PCh. 4.5 - Prob. 104PCh. 4.5 - Prob. 105PCh. 4.5 - Prob. 106PCh. 4.5 - Prob. 107PCh. 4.5 - Prob. 108PCh. 4.5 - Prob. 109RPCh. 4.5 - Prob. 110RPCh. 4.5 - Prob. 111RPCh. 4.5 - Prob. 112RPCh. 4.5 - Prob. 113RPCh. 4.5 - Consider a pistoncylinder device that contains 0.5...Ch. 4.5 - Prob. 115RPCh. 4.5 - Air in the amount of 2 lbm is contained in a...Ch. 4.5 - Air is expanded in a polytropic process with n =...Ch. 4.5 - Nitrogen at 100 kPa and 25C in a rigid vessel is...Ch. 4.5 - Prob. 119RPCh. 4.5 - A mass of 3 kg of saturated liquidvapor mixture of...Ch. 4.5 - A mass of 12 kg of saturated refrigerant-134a...Ch. 4.5 - Prob. 122RPCh. 4.5 - A pistoncylinder device contains helium gas...Ch. 4.5 - Prob. 124RPCh. 4.5 - Prob. 125RPCh. 4.5 - Prob. 126RPCh. 4.5 - Prob. 127RPCh. 4.5 - Water is boiled at sea level in a coffeemaker...Ch. 4.5 - The energy content of a certain food is to be...Ch. 4.5 - Prob. 130RPCh. 4.5 - An insulated pistoncylinder device initially...Ch. 4.5 - An insulated rigid tank initially contains 1.4 kg...Ch. 4.5 - In order to cool 1 ton of water at 20C in an...Ch. 4.5 - A 0.3-L glass of water at 20C is to be cooled with...Ch. 4.5 - A well-insulated 3-m 4m 6-m room initially at 7C...Ch. 4.5 - Prob. 137RPCh. 4.5 - Prob. 138RPCh. 4.5 - Prob. 140RPCh. 4.5 - A pistoncylinder device initially contains 0.35 kg...Ch. 4.5 - Two 10-ft3 adiabatic tanks are connected by a...Ch. 4.5 - Prob. 143RPCh. 4.5 - Prob. 144RPCh. 4.5 - A 3-m3 rigid tank contains nitrogen gas at 500 kPa...Ch. 4.5 - A 0.5-m3 rigid tank contains nitrogen gas at 600...Ch. 4.5 - A well-sealed room contains 60 kg of air at 200...Ch. 4.5 - A room contains 75 kg of air at 100 kPa and 15C....Ch. 4.5 - Prob. 149FEPCh. 4.5 - A pistoncylinder device contains 5 kg of air at...Ch. 4.5 - Prob. 151FEPCh. 4.5 - A 2-kW electric resistance heater submerged in 5...Ch. 4.5 - Prob. 153FEPCh. 4.5 - 1.5 kg of liquid water initially at 12C is to be...Ch. 4.5 - Prob. 155FEPCh. 4.5 - An ordinary egg with a mass of 0.1 kg and a...Ch. 4.5 - Prob. 157FEPCh. 4.5 - A 6-pack of canned drinks is to be cooled from 18C...Ch. 4.5 - Prob. 159FEPCh. 4.5 - An ideal gas has a gas constant R = 0.3 kJ/kgK and...Ch. 4.5 - A pistoncylinder device contains an ideal gas. The...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A 15 cm-OD pipe is buried with its centerline 1.25 m below the surface of the ground [k of soil is 0.35 W/(m K)]. An oil having a density of 800 kg/m³ and a specific heat of 2.1 kJ/(kg K) flows in the pipe at 5.6 L/s. Assuming a ground surface temperature of 5°C and a pipe wall temperature of 95°C, estimate the length of pipe in which the oil temperature decreases by 5.5°C. + Tε = 5ºC Z= 1.25 m D= 15 cm 7p=95°Carrow_forwardFind the solution of the following Differential Equations 1) 4y+y=0, y(0)=2, y'(0) = 0. 2) y+y=0, y(0) = A, y'(0) = B. 3) "+2y'-8y=0, y(0)=1, y'(0)=8. 4) y"-2y-3y=0, y(0)=1, y'(0)=7. 5) y"-ky' =0, y(0)=2, y'(0) =k. 6) y+ky'-2k2y=0, y(0)=2, y'(0) = 2k. 7) y'+4y=0, y(0)=2.8 y+y-17sin(21) y(0)=-1. 9) y-y'-6y=0, y(0)=6. y'(0)=13. 10) y-y=0, 11) y"-4y+4y=0, y(0)=4, y'(0) = 0. y(0) = 2.1, y'(0)=3.9 12) y+2y+2y=0, y(0)=1, y'(0)=-3. 13) "+7y+12y=21e", y(0)=3.5, y'(0)=-10. 14) "+9y=10e", y(0)=0. y'(0) = 0. 15) y+3y+2.25y=91³ +64. y(0)=1, y'(0) = 31.5 16) "-6y+5y= 29 cos(21), y(0)=3.2, y'(0) = 6.2 17) y+2y+2y=0, y(0)=0, y'(0)=1. 18) y+2y+17y=0, y(0)=0, y'(0)=12. 19) y-4y+5y=0, y(0)-1, y'(0) 2. 20) 9y-6y+y=0. y(0)=3, y'(0)=1. 21) -2y+10y=0, y(0)=3, y'(0)=3. 22) 4y-4y+37y=0, (0) 3. y(0) 1.5 23) 4y-8y+5y=0, (0)-0, y(0) 1. 24) y+y+1.25y=0, y(0) 1. y'(0) -0.5 25) y+y=2 cos(1). y(0) 2. y'(0) = 0. 26) -4y+3y=0, (0)-3, y'(0) = 7. 27) y+2y+y=e", y(0)-0. y'(0) = 0. 29) 28) y+2y-3y-10sinh(2),…arrow_forwardNote: Please provide a clear, step-by-step simplified handwritten working out (no explanations!), ensuring it is done without any AI involvement. I require an expert-level answer, and I will assess and rate based on the quality and accuracy of your work and refer to the provided image for more clarity. Make sure to double-check everything for correctness before submitting appreciate your time and effort!. Question:arrow_forward
- 4. Block A and B are two different pieces of wood. Determine the minimum dimension for "a", if the shear stress of the wood is 50Mpa. The thickness of the wood is 30cm. 600N Aarrow_forward1. Determine the reaction force at A. 60 kN 5 B 1 m 1 m- -1 m 4 3 m 30 kN marrow_forwardFind the Laplace Transform of the following functions 1) f() cos(ar) Ans. F(s)=7 2ws 2) f() sin(at) Ans. F(s)= s² + a² 3) f(r)-rcosh(at) Ans. F(s)= 2as 4)(t)=sin(at) Ans. F(s)= 2 5) f(1) = 2te' Ans. F(s)= (S-1) 5+2 6) (1) e cos() Ans. F(s) = (+2)+1 7) (1) (Acostẞr)+ Bsin(Br)) Ans. F(s)- A(s+a)+BB (s+a)+B 8) f()-(-)() Ans. F(s)= 9)(1)(1) Ans. F(s): 10) f(r),()sin() Ans. F(s): 11) 2 k 12) 0 13) 0 70 ㄷ.. a 2a 3a 4a 2 3 4 14) f(1)=1, 0<1<2 15) (1) Ksin(t) 0arrow_forward2. Determine the average normal stress developed in rod AB. The mass is 50kg and the diameter of the rod AB is 8mm. B 8 mmarrow_forward2.64 A 2.75-kN tensile load is applied to a test coupon made from 1.6-mm flat steel plate (E = 200 GPa, v = 0.30). Determine the resulting change in (a) the 50-mm gage length, (b) the width of portion AB of the test coupon, (c) the thickness of portion AB, (d) the cross-sectional area of portion AB. 2.75 kN A 12 mm 50 mm B 2.75 kNarrow_forwardProcedure:1- Cartesian system, 2(D)/(3)D,type of support2- Free body diagram3 - Find the support reactions4- If you find a negativenumber then flip the force5- Find the internal force3D\sum Fx=0\sum Fy=0\sum Fz=0\sum Mx=0\sum My=0\Sigma Mz=02D\Sigma Fx=0\Sigma Fy=0\Sigma Mz=05- Use method of sectionand cut the elementwhere you want to findthe internal force andkeep either side of thesectionarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license